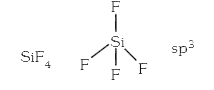
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS|109 VideosINORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS (MATCH THE COLUMN)|2 VideosINORGANIC CHEMISTRY
ALLEN|Exercise PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)|5 VideosHYDROGEN
ALLEN|Exercise EXERCISE-5|17 VideosIonic Equilibrium
ALLEN|Exercise All Questions|37 Videos
Similar Questions
Explore conceptually related problems
ALLEN-INORGANIC CHEMISTRY-CHEMICAL BONDING
- Number of carbon atom present linearly in C(3)O(2) :-
Text Solution
|
- In which pair both are not isostructural :-
Text Solution
|
- In which of the following molecule all bond length are equal :-
Text Solution
|
- Which of the following set do not have sp^(3)d hybridization :-
Text Solution
|
- Which of the following has different hybridization than other :-
Text Solution
|
- Consider the following compounds and select the incorrect statement fr...
Text Solution
|
- Which of the following molecule exhibit P(pi)-P(pi) bonding?
Text Solution
|
- Which of the following statement is not correct
Text Solution
|
- Select in which both have sea-saw shape-
Text Solution
|
- Which of the following have same geometry -
Text Solution
|
- In which hybridisation, resulting all orbitals are NOT equivalent-
Text Solution
|
- Out of CHCl(3), CH(4) and SF(4) the molecules do not having regular ge...
Text Solution
|
- The correct order of increasing s-character (in percentage) in the hyb...
Text Solution
|
- Which of the following will have pyramidal shape :-
Text Solution
|
- Which of the following is electron deficient compound :-
Text Solution
|
- Maximum number of identical bond length are present in
Text Solution
|
- Match column-I with column-II and select the correct answer :-
Text Solution
|
- In which type of molecule, the dipole moment will be nonzero :-
Text Solution
|
- Which of the following compounds give paramagnetic gas on decompositio...
Text Solution
|
- Arrange the following compound in order of increasing dipole moment : ...
Text Solution
|