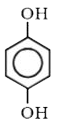
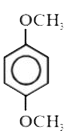
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
INORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS|109 VideosINORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS (MATCH THE COLUMN)|2 VideosINORGANIC CHEMISTRY
ALLEN|Exercise PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)|5 VideosHYDROGEN
ALLEN|Exercise EXERCISE-5|17 VideosIonic Equilibrium
ALLEN|Exercise All Questions|37 Videos
Similar Questions
Explore conceptually related problems
ALLEN-INORGANIC CHEMISTRY-CHEMICAL BONDING
- Which of the following molecule is planar as well as polar :
Text Solution
|
- Which of the following compound have number of p pi-p pi bond equal t...
Text Solution
|
- Which of the following molecule has same dipole moment :-
Text Solution
|
- Which of the following species is non polar with presence of polar bon...
Text Solution
|
- Which of the following species are hypervalent ? (I) ClO(4)^(-) " ...
Text Solution
|
- {:("Bond" ,"Bond dissociation energy" ("kJ mole"^(–1))),( C-I ,240 " ...
Text Solution
|
- Which have maximum number of lone pair on central atom :-
Text Solution
|
- Total no. of vacant orbitals in valence shell of sulphur when it under...
Text Solution
|
- Indicate the wrong statement :-
Text Solution
|
- Which of the following statement is incorrect :-
Text Solution
|
- If compound AX(3) is a hypervalent compound then the group no of elem...
Text Solution
|
- Correct order of extent of overlapping is :-
Text Solution
|
- Which is formed in II^(nd) excited state :-
Text Solution
|
- When two A.O. combine they form B.MO & ABMO. During BMO .x. energy is ...
Text Solution
|
- Dative Bond is present in :-
Text Solution
|
- Which of the following element never from compound in ground state : -
Text Solution
|
- Which of the following pairs have same hybridisation ?
Text Solution
|
- Which orbital is not involved in the formation of PCl(5) molecule :-
Text Solution
|
- Shape of molecule having 4-bond pair and one lone pair is :-
Text Solution
|
- Which of the following molecules has both p(pi)-p(pi) and p(pi)-d(pi) ...
Text Solution
|