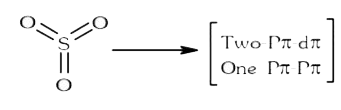
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS|109 VideosINORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS (MATCH THE COLUMN)|2 VideosINORGANIC CHEMISTRY
ALLEN|Exercise PERIODIC TABLE (MATCH THE COLUMN TYPE QUESTIONS)|5 VideosHYDROGEN
ALLEN|Exercise EXERCISE-5|17 VideosIonic Equilibrium
ALLEN|Exercise All Questions|37 Videos
Similar Questions
Explore conceptually related problems
ALLEN-INORGANIC CHEMISTRY-CHEMICAL BONDING
- Which orbital is not involved in the formation of PCl(5) molecule :-
Text Solution
|
- Shape of molecule having 4-bond pair and one lone pair is :-
Text Solution
|
- Which of the following molecules has both p(pi)-p(pi) and p(pi)-d(pi) ...
Text Solution
|
- Select the correct statement
Text Solution
|
- Species present in acidic aq. solution are :
Text Solution
|
- Intramolecular hydrogen bonding is not present in :-
Text Solution
|
- Which of the following order of H-bond strength is not correct :-
Text Solution
|
- Dipole-dipole (Keesom) attraction is present in :-
Text Solution
|
- The solubility of inert gases in water is due to
Text Solution
|
- London Dispersion force is present between :-
Text Solution
|
- The stability of species Li(2),Li(2)^(-)and Li(2)^(+) increases in the...
Text Solution
|
- Which of the following compound donot exist?
Text Solution
|
- Among the following, VWF are maximum in :-
Text Solution
|
- Which of the following can not be calculated by Born Haber cycle forma...
Text Solution
|
- Which pair is not isomorphous pair :-
Text Solution
|
- Which of the following compound form non conducting solution in water....
Text Solution
|
- How many compounds show conductivity HCl(aq), HCl (molten), AlCl(3) (m...
Text Solution
|
- In which of the following sets, all the three compounds have bonds tha...
Text Solution
|
- Which of the following B.P. order is not correct
Text Solution
|
- Strength of H-bond and boiling point order is opposite for :-
Text Solution
|