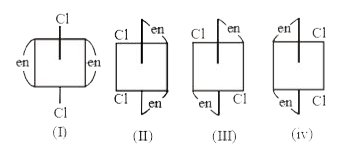
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS (MATCH THE COLUMN)|2 VideosINORGANIC CHEMISTRY
ALLEN|Exercise p-BLOCK ELEMENT|80 VideosINORGANIC CHEMISTRY
ALLEN|Exercise CHEMICAL BONDING|119 VideosHYDROGEN
ALLEN|Exercise EXERCISE-5|17 VideosIonic Equilibrium
ALLEN|Exercise All Questions|37 Videos
Similar Questions
Explore conceptually related problems
ALLEN-INORGANIC CHEMISTRY-COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS
- Which of following isomers of [M(NH(3))(2)Cl(2)] would react with sil...
Text Solution
|
- Which of the following complex will follow EAN rule ?
Text Solution
|
- Identify the geometrical isomers of the following:-
Text Solution
|
- Which of the following will show optical isomers? (I) cis-[Co(NH(3))...
Text Solution
|
- Which of the following isomerism is/are not shown by the complex [Co(C...
Text Solution
|
- Three arrangements are shown for the complex [Co(e n) (NH(3))(2)Cl(2)]...
Text Solution
|
- Which kind of isomerism is exhibited by octahedral [Co(NH(3))(2)Br(2)]...
Text Solution
|
- Which of the following has an option isomer ?
Text Solution
|
- Which of the following pairs represents linkage isomers ?
Text Solution
|
- Select the correct statement from the following?
Text Solution
|
- [Fe(e n)(2)(H(2)O)(2)]^(2+) +e n to complex (x). The correct statement...
Text Solution
|
- Which of the following complexes show geometrical as well as optical i...
Text Solution
|
- The total number of possible isomers for the complex compound [Cu^(II)...
Text Solution
|
- [Co(e n)(3)]^(3+) ion is expected to show :-
Text Solution
|
- How many geometrical isomers are there for [Co(NH(3))(2)Cl(4)]^(-), a...
Text Solution
|
- How many isomers are possible for the complex ion [Cr(NH(3))(3)Cl(3)] ...
Text Solution
|
- For the square planar complex [Pt(NH(3))(NH(2)OH)(py)(No(2))]^(o+) how...
Text Solution
|
- Isomerisms exhibited by [Cr(NH3)2(H2O)2Cl2]^+ are
Text Solution
|
- Which of the following is true for the complex Co(NO(2) )(Cl)(2) .5NH(...
Text Solution
|
- The total No. of isomers possible for the complex [Co (e n)(2)Cl(2)] i...
Text Solution
|