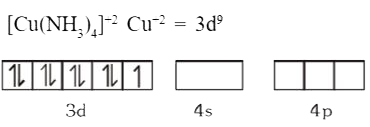
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
INORGANIC CHEMISTRY
ALLEN|Exercise COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS (MATCH THE COLUMN)|2 VideosINORGANIC CHEMISTRY
ALLEN|Exercise p-BLOCK ELEMENT|80 VideosINORGANIC CHEMISTRY
ALLEN|Exercise CHEMICAL BONDING|119 VideosHYDROGEN
ALLEN|Exercise EXERCISE-5|17 VideosIonic Equilibrium
ALLEN|Exercise All Questions|37 Videos
Similar Questions
Explore conceptually related problems
ALLEN-INORGANIC CHEMISTRY-COORDINATION COMPOUNDS & d-BLOCK COMPOUNDS
- The crystal field -splitting for Cr^(3+) ion in octahedral field chang...
Text Solution
|
- Which of the following is the high spin complex?
Text Solution
|
- Which has maximum paramagnetic nature ?
Text Solution
|
- Which of the following order of CFSE is incorrect?
Text Solution
|
- Which of the following is/are inner orbital complex as well as dimagne...
Text Solution
|
- Which of the following is/are correct about [Cu(NH(3))(4)]SO(4) :
Text Solution
|
- The hybridisation of [CoF(6)]^(3-) & [Co(C(2)O(4))(3)]^(3-) are :-
Text Solution
|
- A complex of a certain metal ion has a magnetic moment of 4.90BM Anoth...
Text Solution
|
- [Sc (H(2)O)(6)]^(3+) ion is :-
Text Solution
|
- Which of the following complex compounds does not exhibits cis-trans i...
Text Solution
|
- Which of the following correct?
Text Solution
|
- Of the following complex ions, the one that probably has the largest o...
Text Solution
|
- Which is not a pi-bonded complex ?
Text Solution
|
- What is wrong about the compound K[Pt (eta^(2)-C(2)H(4))Cl(3)]
Text Solution
|
- Which of the following order is not correct?
Text Solution
|
- Which of the following is correct for zeise.s salt ?
Text Solution
|
- Which amongst the following are organometalic componds :- (i) Al(2)(...
Text Solution
|
- Which amongst the following metal carbonyls are inner orbital complex ...
Text Solution
|
- To an acidified dichromate solution, a pinch of Na(2)O(2) is added and...
Text Solution
|
- Pick out the incorrect statement.
Text Solution
|