A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
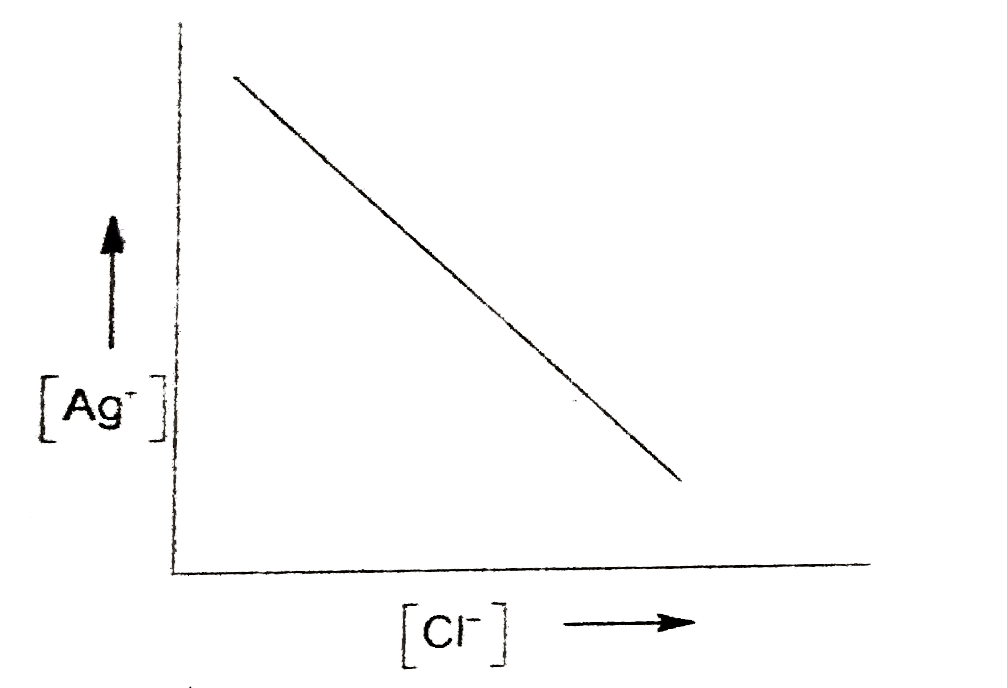
- In a saturated solution of AgCl, NaCl is added gradually. The concentr...
Text Solution
|
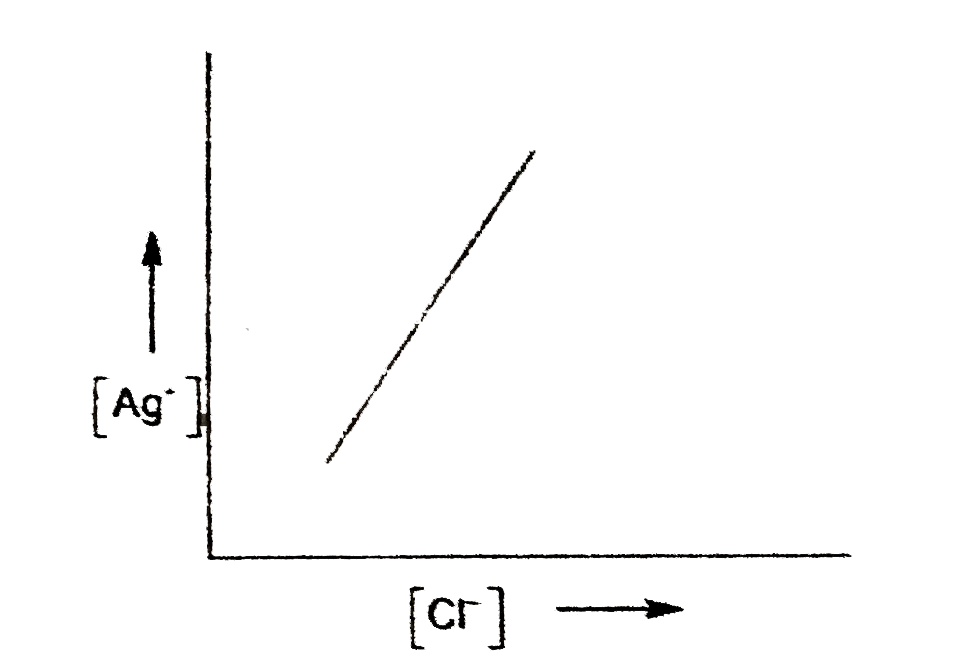
- In a saturated solution of AgCl, NaCl is added gradually the concentra...
Text Solution
|
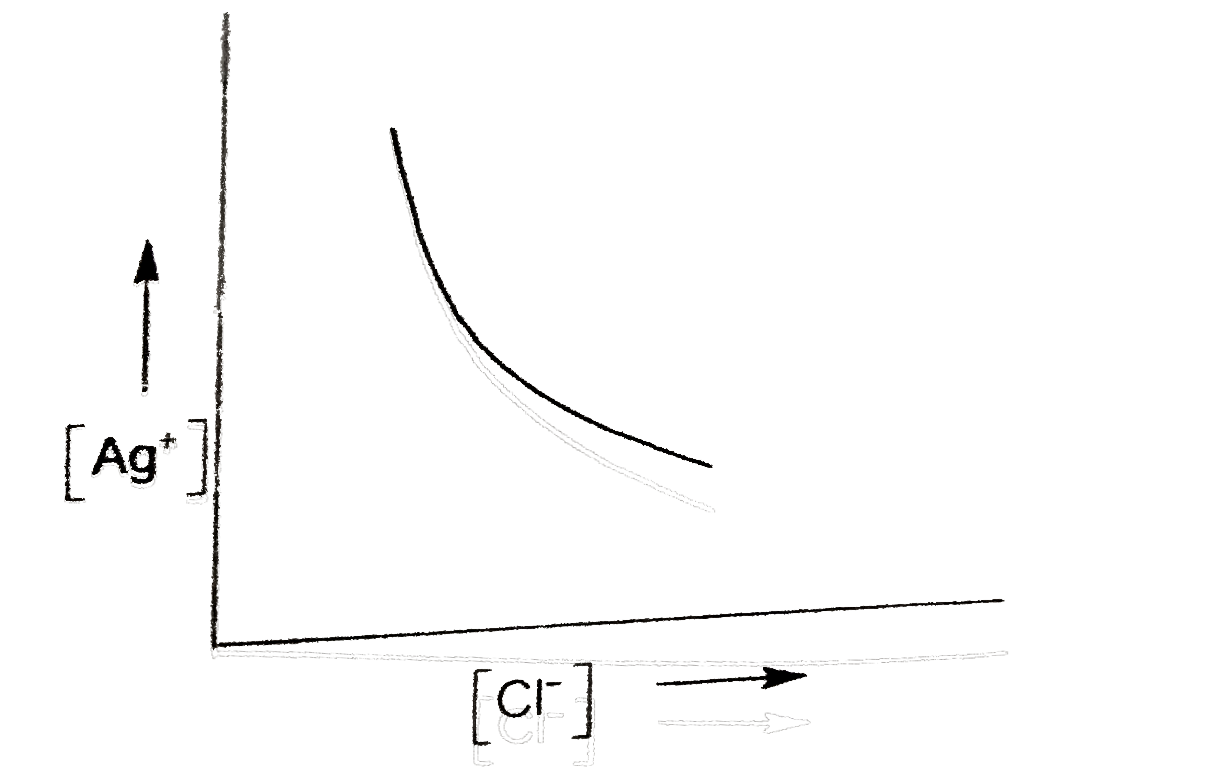
- In a saturated solution of AgCl, NaCl is added gradually. The concentr...
Text Solution
|
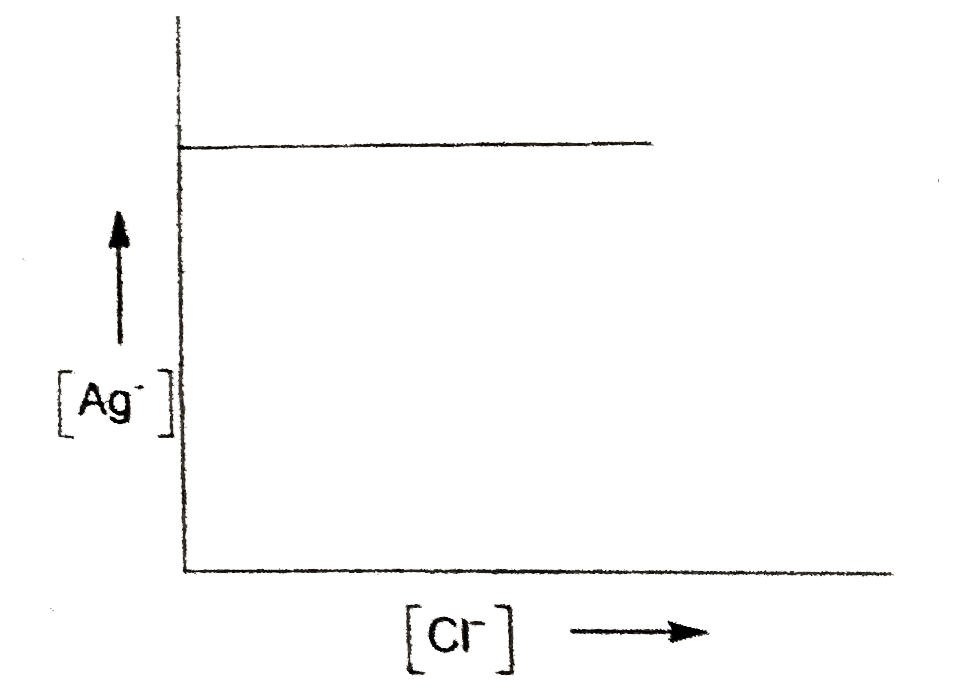
- The concentration of Ag^(+) ions in a given saturated solution of AgCl...
Text Solution
|
- what must be the concentration of aqueous of NH(3) which must be added...
Text Solution
|
- Excess of AgCl is added to 0.1 M solution of KBr at 298 K. Calculate t...
Text Solution
|
- NaCl solution is added to a saturated solution of PbCl(2). What will h...
Text Solution
|
- NaCl solution is added to a saturated solution of PbCl(2). What will h...
Text Solution
|
- In 1 L saturated solution of AgCl [K(sp)(AgCl)=1.6xx10^(-10)], 0.1 " m...
Text Solution
|