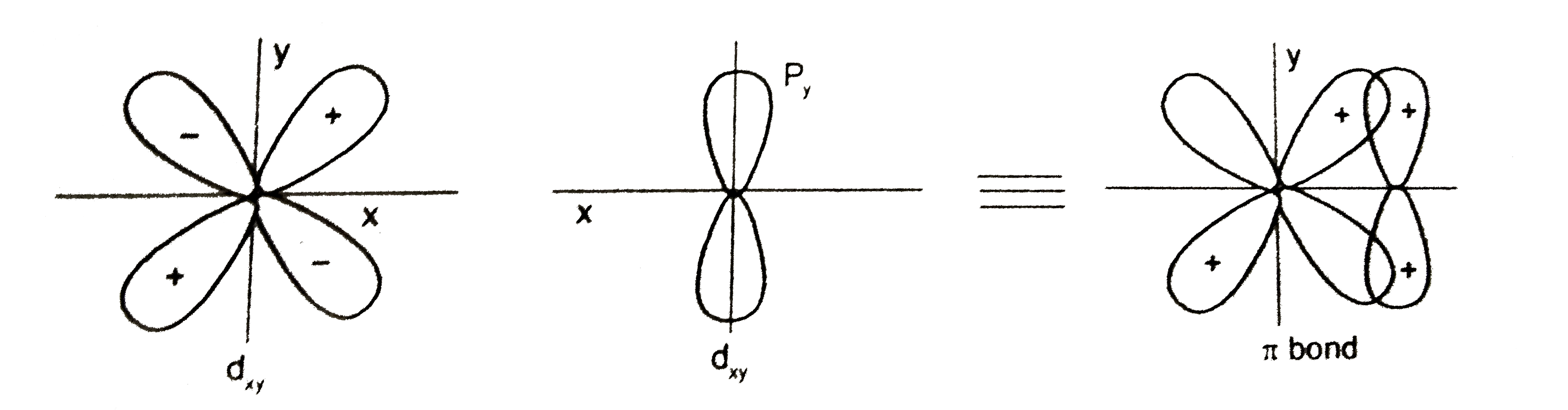
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider a p(y)-orbital of an atom and identify correct statement.
Text Solution
|
- Consider a P(y) orbital of an atom and identify correct statement
Text Solution
|
- The correct statement regarding the third orbit of hydrgen atom is
Text Solution
|
- Identify the correct statement (s) regarding 3p(z) orbital.
Text Solution
|
- How do p(x),p(y) and p(z) atomic orbitals differ ?
Text Solution
|
- Consider the following statement regarding seed germination and ident...
Text Solution
|
- Consider the following three orbitals : Correct statement(s) rega...
Text Solution
|
- Consider the following atomic orbitals : Which of the following s...
Text Solution
|
- Out of p(x), p(y), p(z) orbitals which p orbital takes part in sp hybr...
Text Solution
|