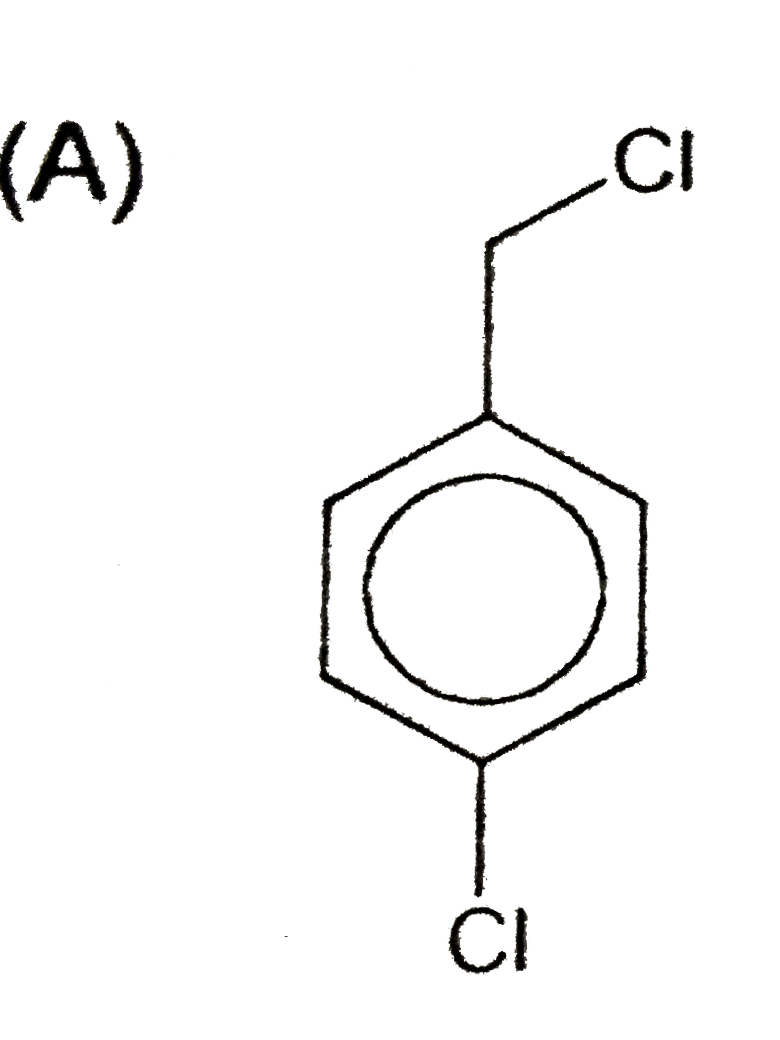
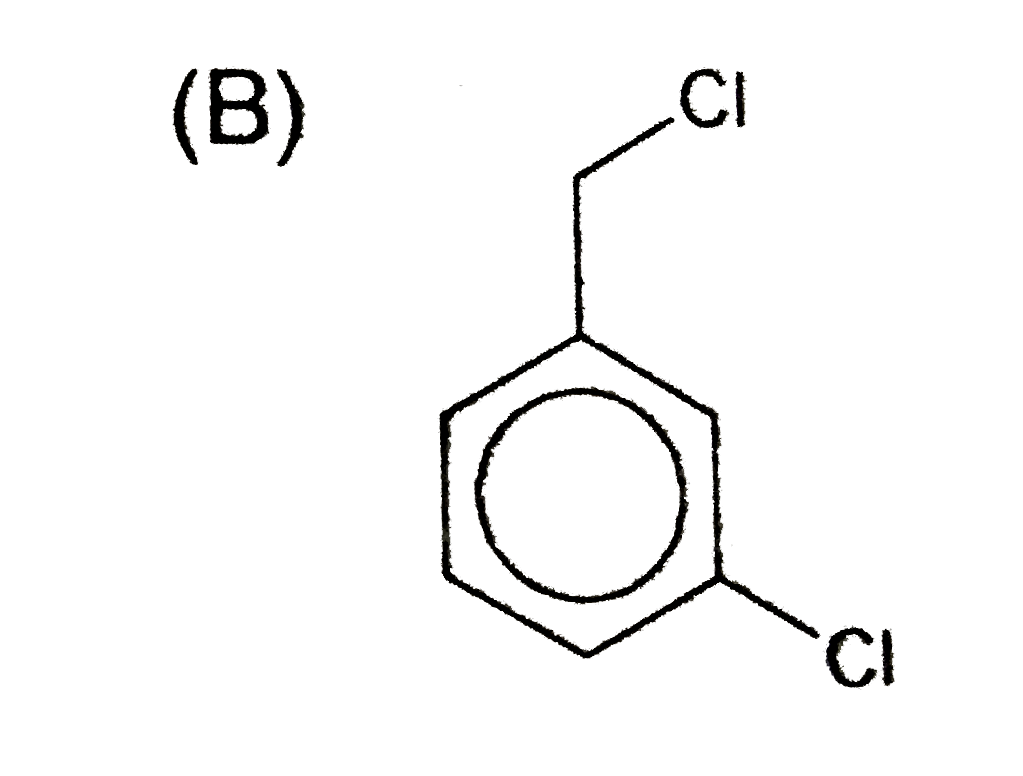
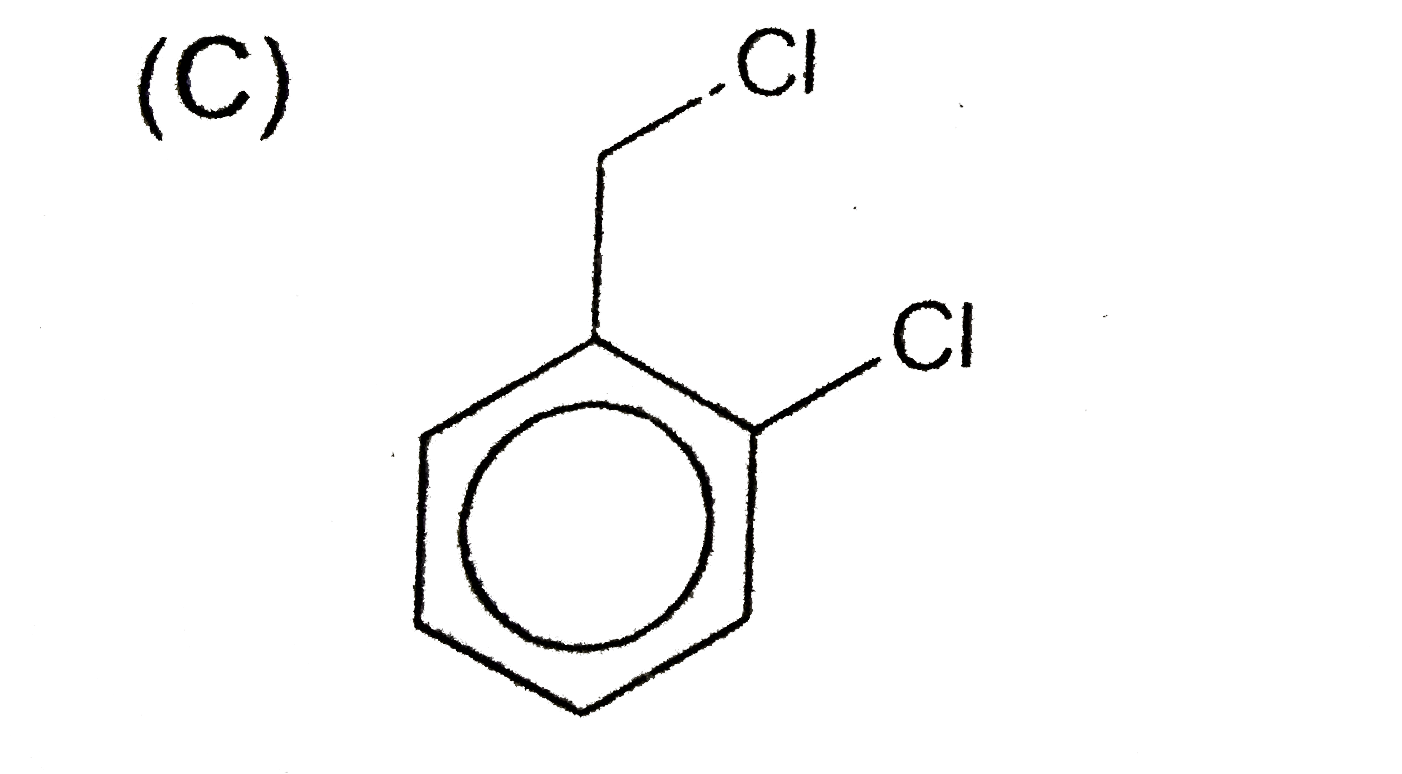
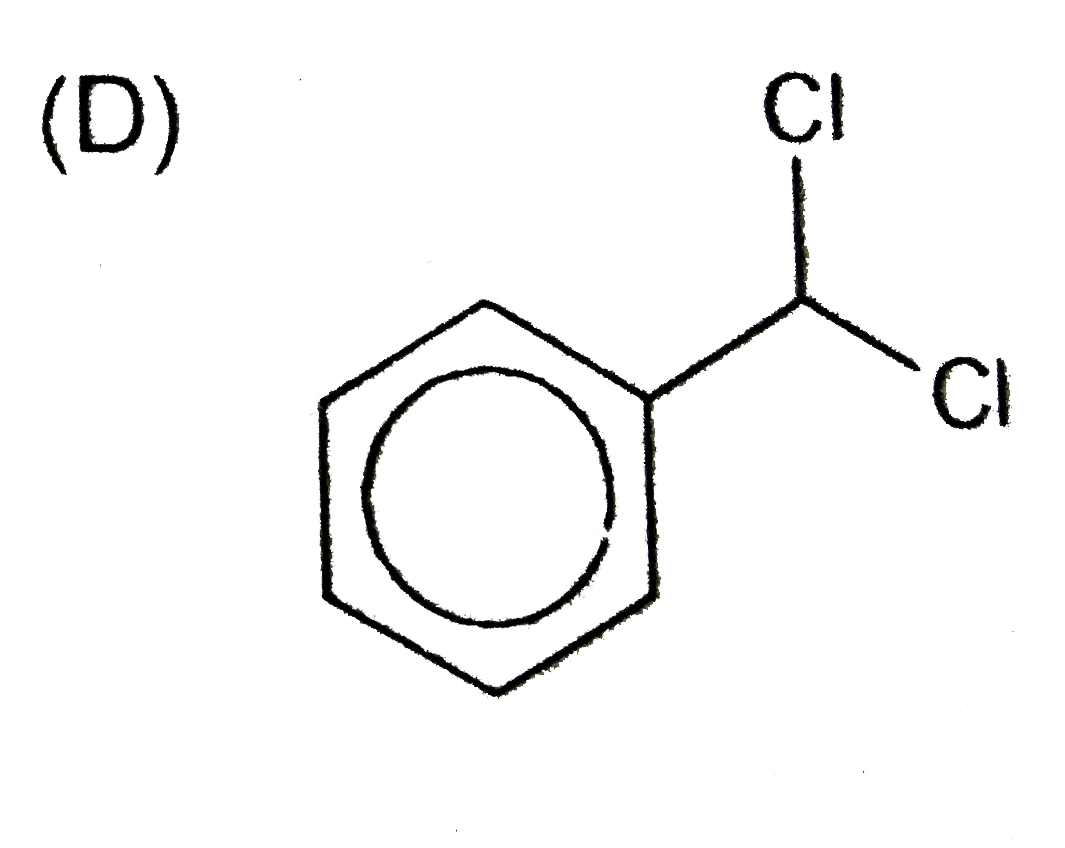
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
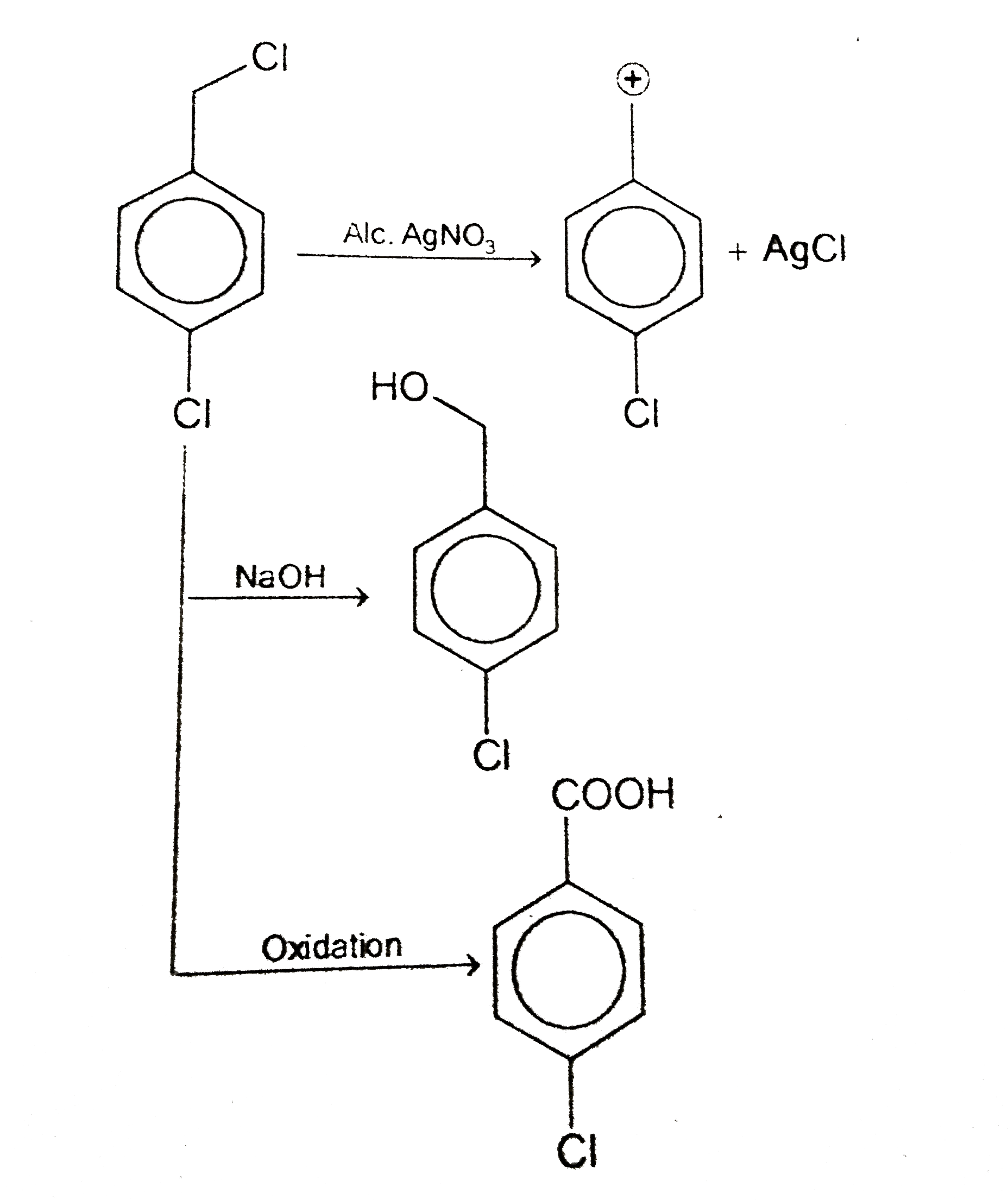
- An aromatic compound C(6)H(6)Cl(2)(A), gives AgCl on boiling with alco...
Text Solution
|
- An organic compound (A) (C(5)H(7)OCl) reacts rapidly with ethanol to g...
Text Solution
|
- C(6)H(6)CI(6) on treatment with alcoholic KOH yields .
Text Solution
|
- An aromatic compound (A), C(7),H(6)Cl(2) gives AgCl on boiling with al...
Text Solution
|
- Compound (A) C(8)H(6)O(2) on treatment with aq. NaOH followed by acidi...
Text Solution
|
- An aromatic compound 'A' (Molecular formula C(8)H(8)O) ) gives positiv...
Text Solution
|
- An organic compound A (C(7)H(6)Cl(2)) on treatment with NaOH solution ...
Text Solution
|
- An aromatic compound C(7)H(7)Cl on oxidation gives another aromatic co...
Text Solution
|
- An organic compound (A) C(7)H(15)CI on treatment with alcoholic causti...
Text Solution
|