Which of the following options is incorrect?
Which of the following options is incorrect?
A
`pK_(a_(1))` cis but-2-ene dioic acid gt trans but-2-ene dioic acid
B
dipole moment 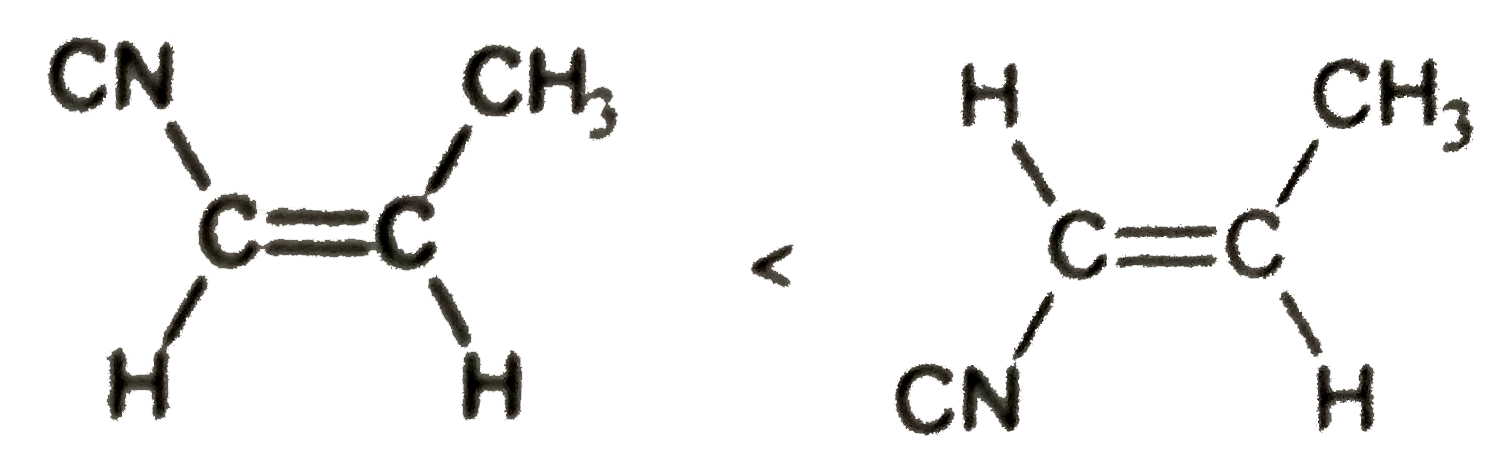
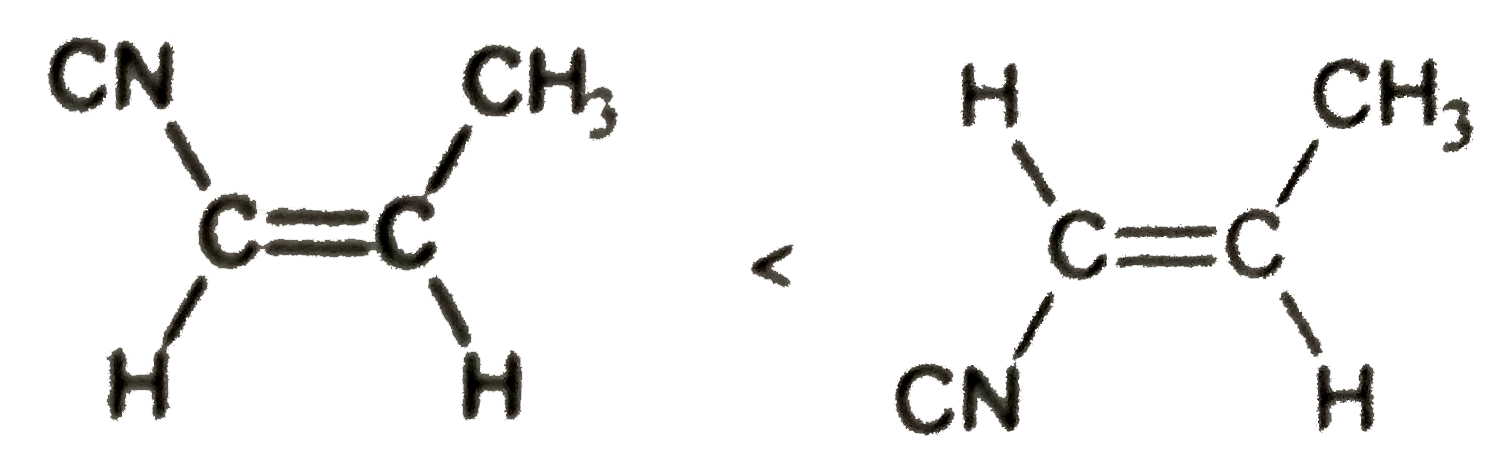
C
Stability 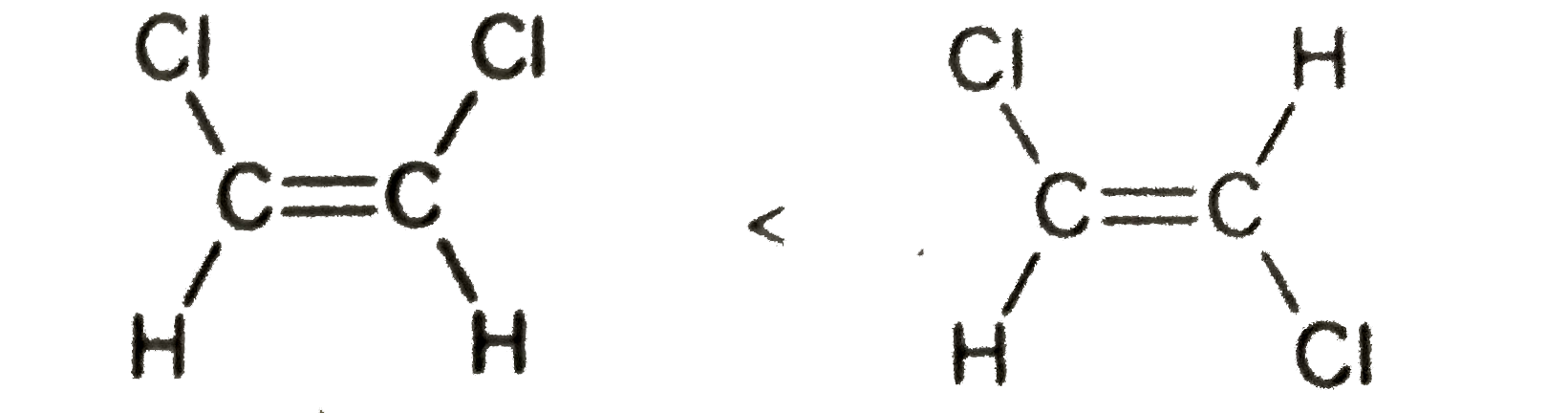
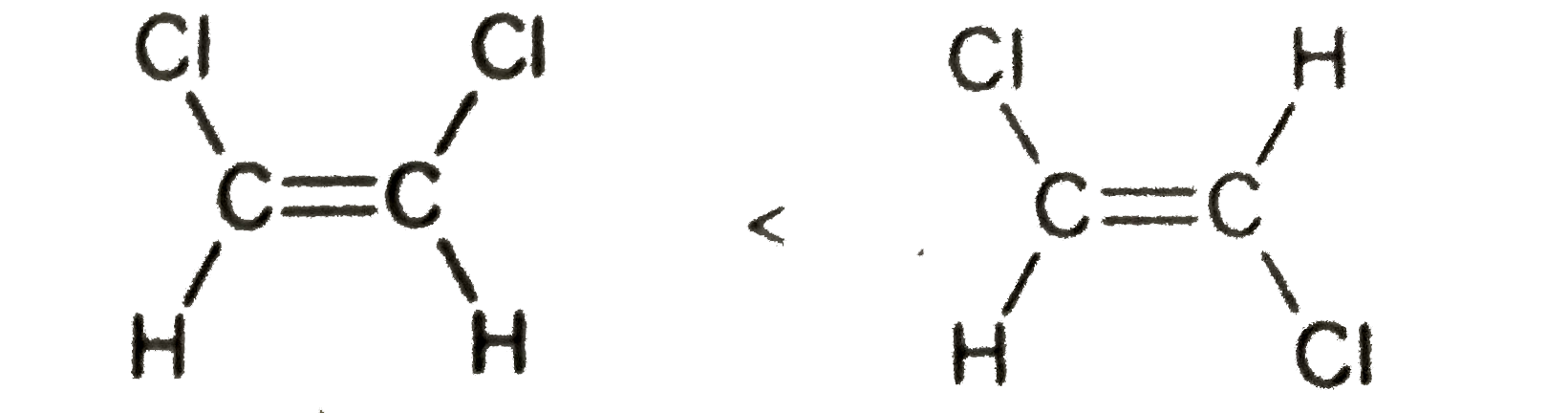
D
Basicity 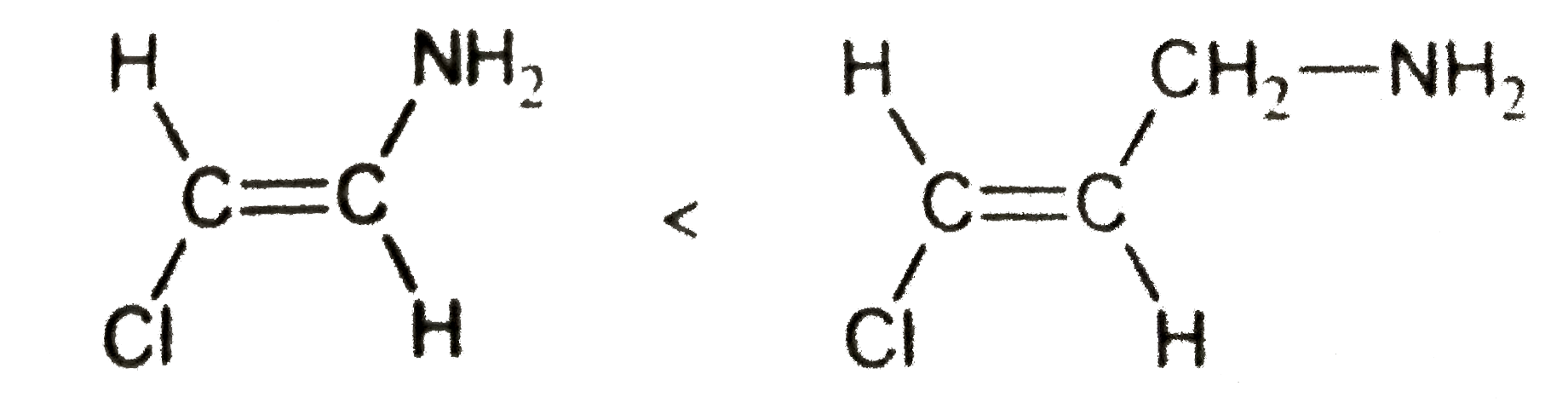
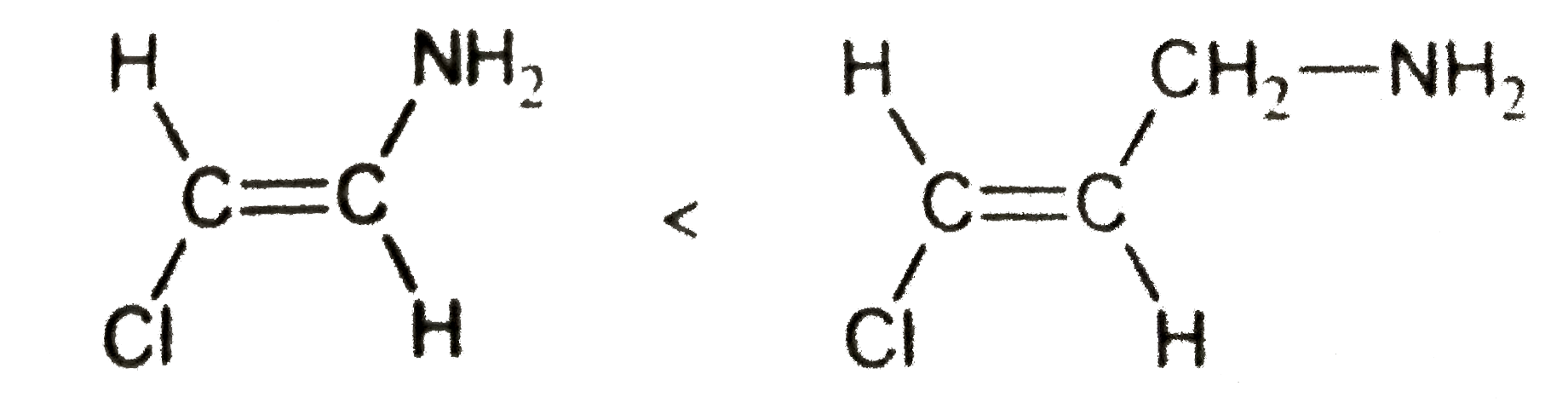
Text Solution
AI Generated Solution
The correct Answer is:
To solve the question of identifying the incorrect statement among the given options, we will analyze each option step-by-step.
### Step 1: Analyze Option A
**Statement:** The pKa value of cis-but-2-ene dioic acid is greater than that of trans-but-2-ene dioic acid.
- **Explanation:**
- In cis-but-2-ene dioic acid, intramolecular hydrogen bonding occurs due to the proximity of the carboxylic acid groups. This bonding stabilizes the molecule, making it less acidic, which results in a higher pKa value.
- In trans-but-2-ene dioic acid, there is no such intramolecular hydrogen bonding, leading to greater acidity and thus a lower pKa value.
- **Conclusion:** This statement is correct.
### Step 2: Analyze Option B
**Statement:** The dipole moment of cis-but-2-ene is greater than that of trans-but-2-ene.
- **Explanation:**
- In cis-but-2-ene, the polar groups (like CH3 and CN) are on the same side, which adds to the overall dipole moment.
- In trans-but-2-ene, these groups are on opposite sides, which tends to cancel out their dipole moments, resulting in a lower overall dipole moment.
- **Conclusion:** This statement is correct.
### Step 3: Analyze Option C
**Statement:** The stability order of cis and trans dichloroethene is such that trans is more stable than cis.
- **Explanation:**
- The cis form of dichloroethene has chlorine atoms closer together, which can lead to steric hindrance and repulsion. However, it can also allow for intramolecular interactions that can stabilize the molecule.
- The trans form has the chlorine atoms further apart, which reduces steric hindrance but does not allow for the same intramolecular attractions.
- Generally, it is accepted that the trans form is more stable due to less steric hindrance, but the argument presented in the video suggests that the cis form may have stabilizing interactions that are not considered in the traditional view.
- **Conclusion:** This statement is incorrect based on the argument presented in the video.
### Step 4: Analyze Option D
**Statement:** The basicity of the amine group is greater when attached to the vinylic carbon than when attached to the allylic carbon.
- **Explanation:**
- When the amine group is attached to the vinylic carbon, its lone pairs can participate in resonance, making them less available for protonation and thus less basic.
- When attached to the allylic carbon, the lone pairs are not involved in resonance with the double bond, making them more available for protonation and thus more basic.
- **Conclusion:** This statement is correct.
### Final Conclusion
After analyzing all the options, we find that the incorrect statement is **Option C**.
To solve the question of identifying the incorrect statement among the given options, we will analyze each option step-by-step.
### Step 1: Analyze Option A
**Statement:** The pKa value of cis-but-2-ene dioic acid is greater than that of trans-but-2-ene dioic acid.
- **Explanation:**
- In cis-but-2-ene dioic acid, intramolecular hydrogen bonding occurs due to the proximity of the carboxylic acid groups. This bonding stabilizes the molecule, making it less acidic, which results in a higher pKa value.
- In trans-but-2-ene dioic acid, there is no such intramolecular hydrogen bonding, leading to greater acidity and thus a lower pKa value.
...
Similar Questions
Explore conceptually related problems
Which of the following option is incorrect?
A 5 kg block rests on a horizontal surface. Which of the following option is incorrect? ( g=10 m/s )
Matching the imformation given in the three columns of the following table. {:("Column 1"," ""Column 2"," ""Column 3"),("(Metals)","(Name of Ore)","Name of composition of Ore"),("(I) Cu","(i) Glauberite","(P) Nitre"),("(II) K","(ii) Chlorargyrite","(Q) Glauber's salt"),("(III) Ag","(iii) Indian salt petre","(R) Fool's gold"),("(IV) Na","(iv) Chalcopyrite","(S) Horn silver"):} Which of the following option is incorrect ?
Matching the imformation given in the three columns of the following table. {:("Column 1","Column 2","Column 3"),("(Reaction)","(Process)","Ores(Reactant)"),((I)PbS rarr PbO,(i) "Roasting","(P) Zinc blende "),((II)CaCO_(3) rarr CaO,(ii)"Calcination","(Q) Galena "),((III) ZnS rarr Zn,(iii) "Carbon reduction","(R) Pyrite"),((IV) CuFeS_(2) rarr Ca,(iv) "Self reduction",(S) "Limestone"):} Which of the following option is incorrect ?
Which of the following option is incorrect about the larynx (sound box) ?
Which of the following option is incorrect w.r.t. to the anemophily
A projectile of mass 1kg is projected with a velocity of sqrt(20)m//s such that it strikes on the same level as the point of projection at a distance of sqrt(3)m . Which of the following options are incorrect:
Which one of the following options is incorrect ?
Which one of the following option is incorrect ?
Which one of the following options is incorrect ?
Recommended Questions
- Which of the following options is incorrect?
Text Solution
|
- Which one of the following options is incorrect ?
Text Solution
|
- Which of the following option(s) is/are incorrect?
Text Solution
|
- Which one of the following option is incorrect ?
Text Solution
|
- Which of the following is incorrect option
Text Solution
|
- Which of the following options are incorrect.
Text Solution
|
- Which of the following options is the only INCORRECT combination ?
Text Solution
|
- Which one of the following options is incorrect?
Text Solution
|
- Which of the following combination is INCORRECT option ?
Text Solution
|