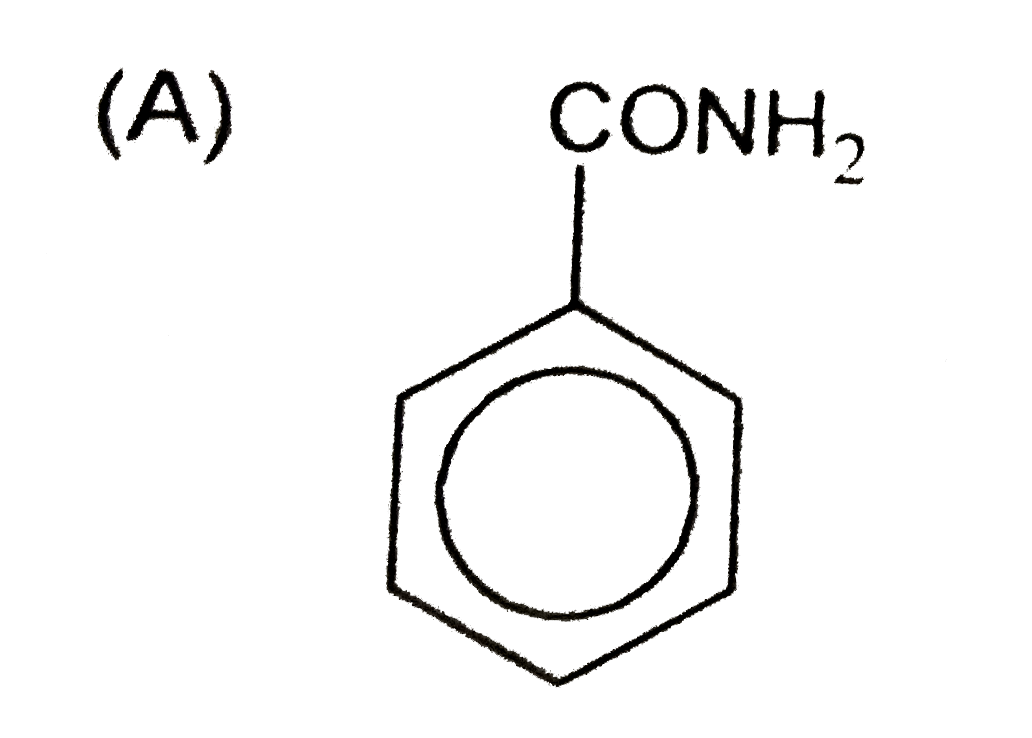
A
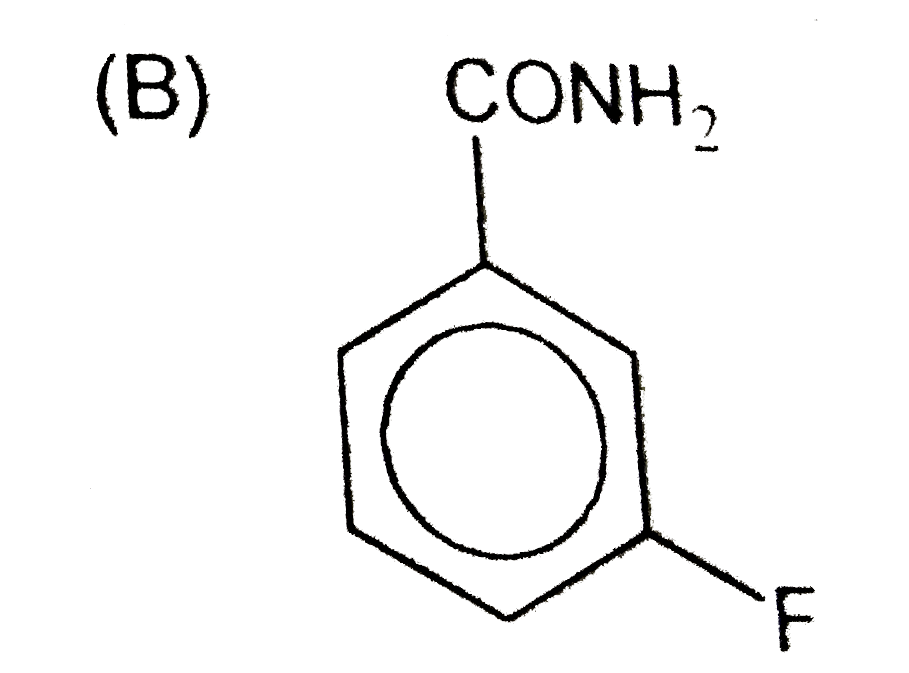
B
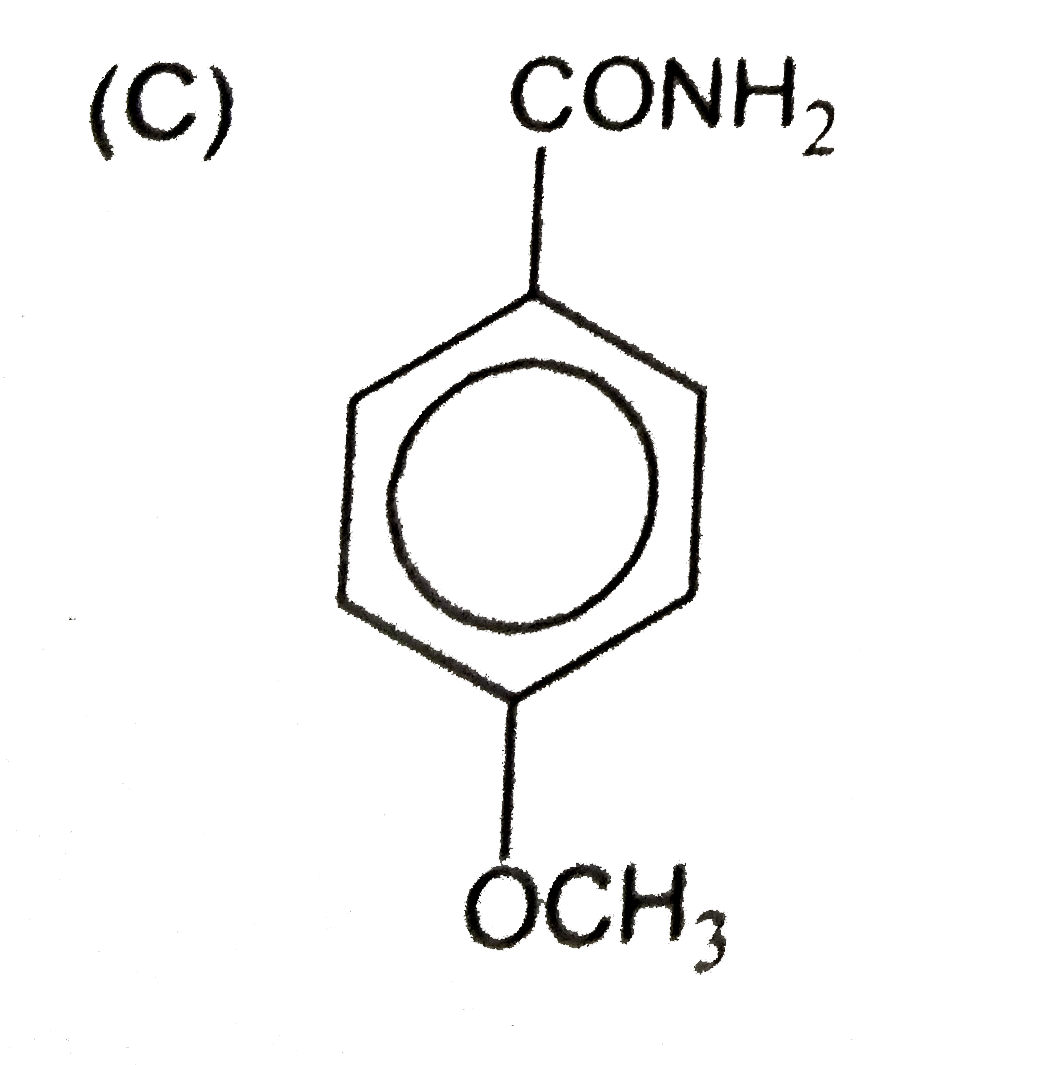
C
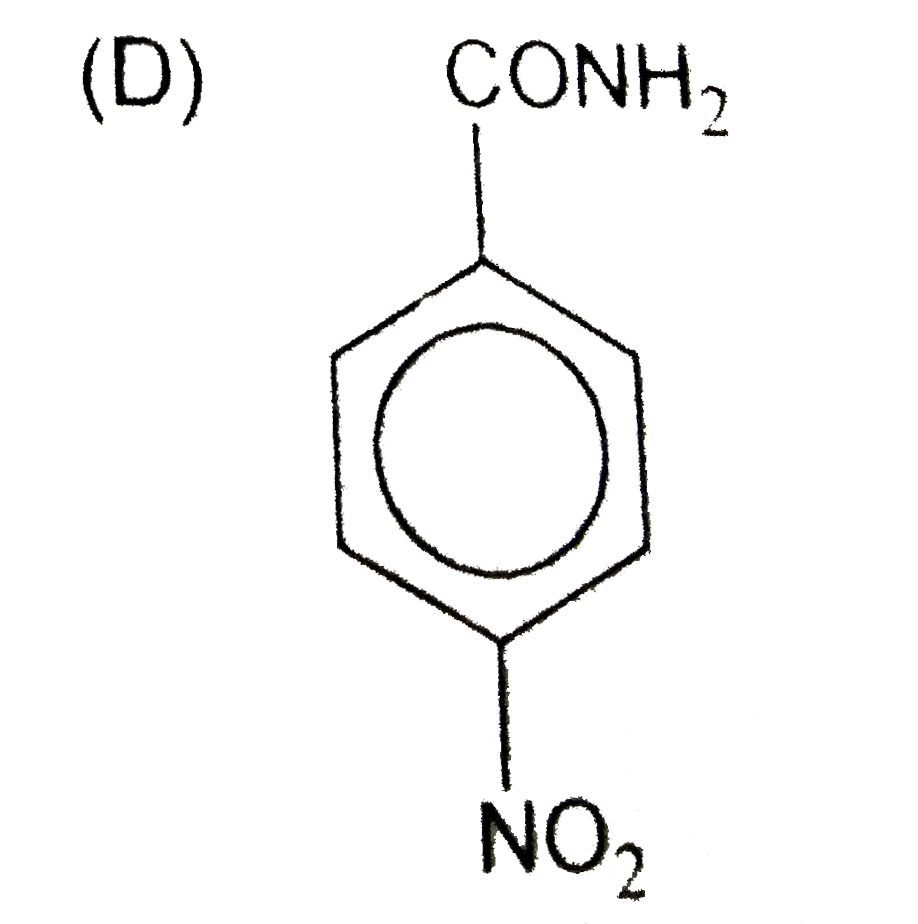
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following can undergo hofmann brimamide reaction most eas...
Text Solution
|
- Which of the following undergoes Hydrolysis most easily:
Text Solution
|
- Which of the following undergoes Hydrolysis most easily:
Text Solution
|
- Which of the following compound undergo hydrolysis most easily :
Text Solution
|
- Which of the following amides will not undergo Hofmann bromamide react...
Text Solution
|
- Which of the following compounds undergoes mucleophilic substitution r...
Text Solution
|
- Which the following compound undergoes nucleophilic substitution react...
Text Solution
|
- Which of the following amides will not undergo Hofmann bromami...
Text Solution
|
- Which of the following compounds undergoes nucleophilic substance rea...
Text Solution
|