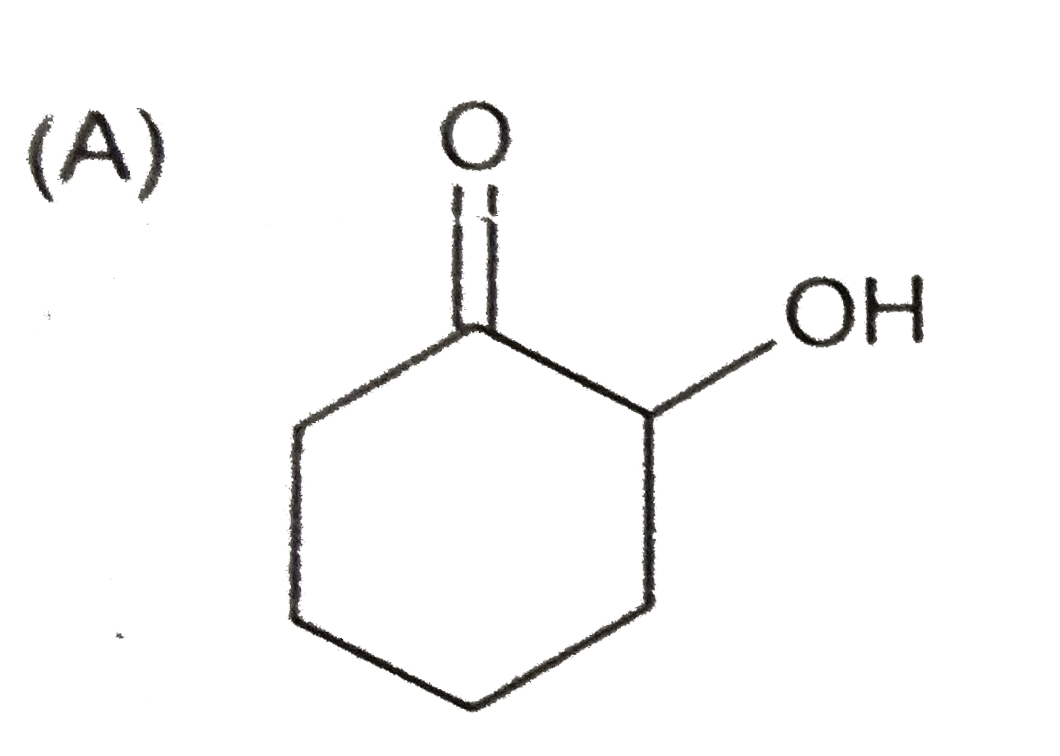
A
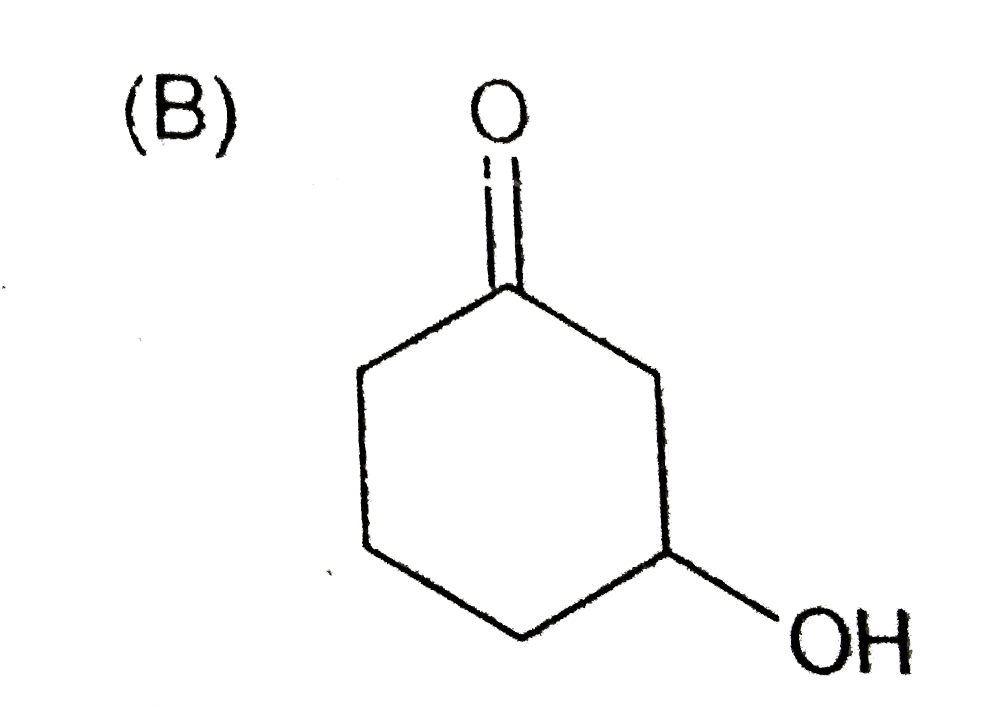
B
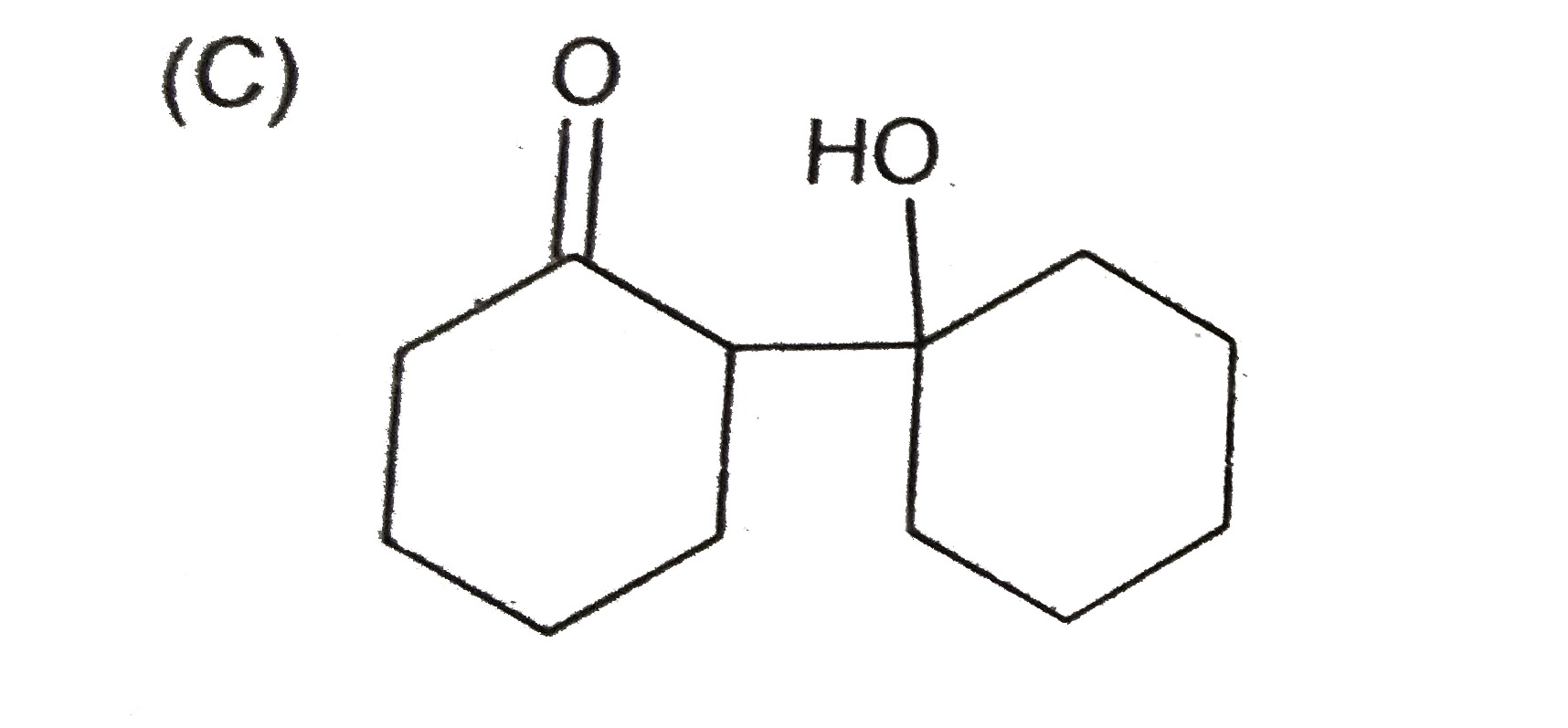
C
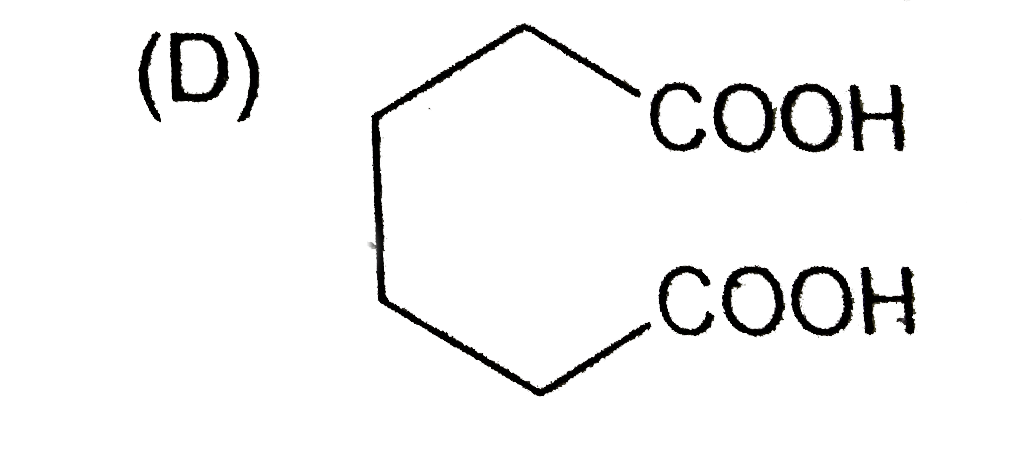
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When cyclohexanone is treated with Na(2)CO(3) soln, we get
Text Solution
|
- When SO(2) is passed into an Na(2)CO(3) solution, it produces
Text Solution
|
- When CO(2) is passed into brine solution saturated with ammonia we get
Text Solution
|
- STEP-(I): Na(2)CO(3)(aq)overset("excess" SO(2))rarr 'A' overset(Na(2)C...
Text Solution
|
- When an ether is treated with P2S5 we get :
Text Solution
|
- When acetamide is treated with Br(2) and caustic soda, then we get
Text Solution
|
- Cyclohexanone is treated with Ba(OH) (2) gives
Text Solution
|
- When ethyl alcohol is treated with Cl2 we get
Text Solution
|
- When Grignard reagent (CH(3) MgBr) is treated with water , we ...
Text Solution
|