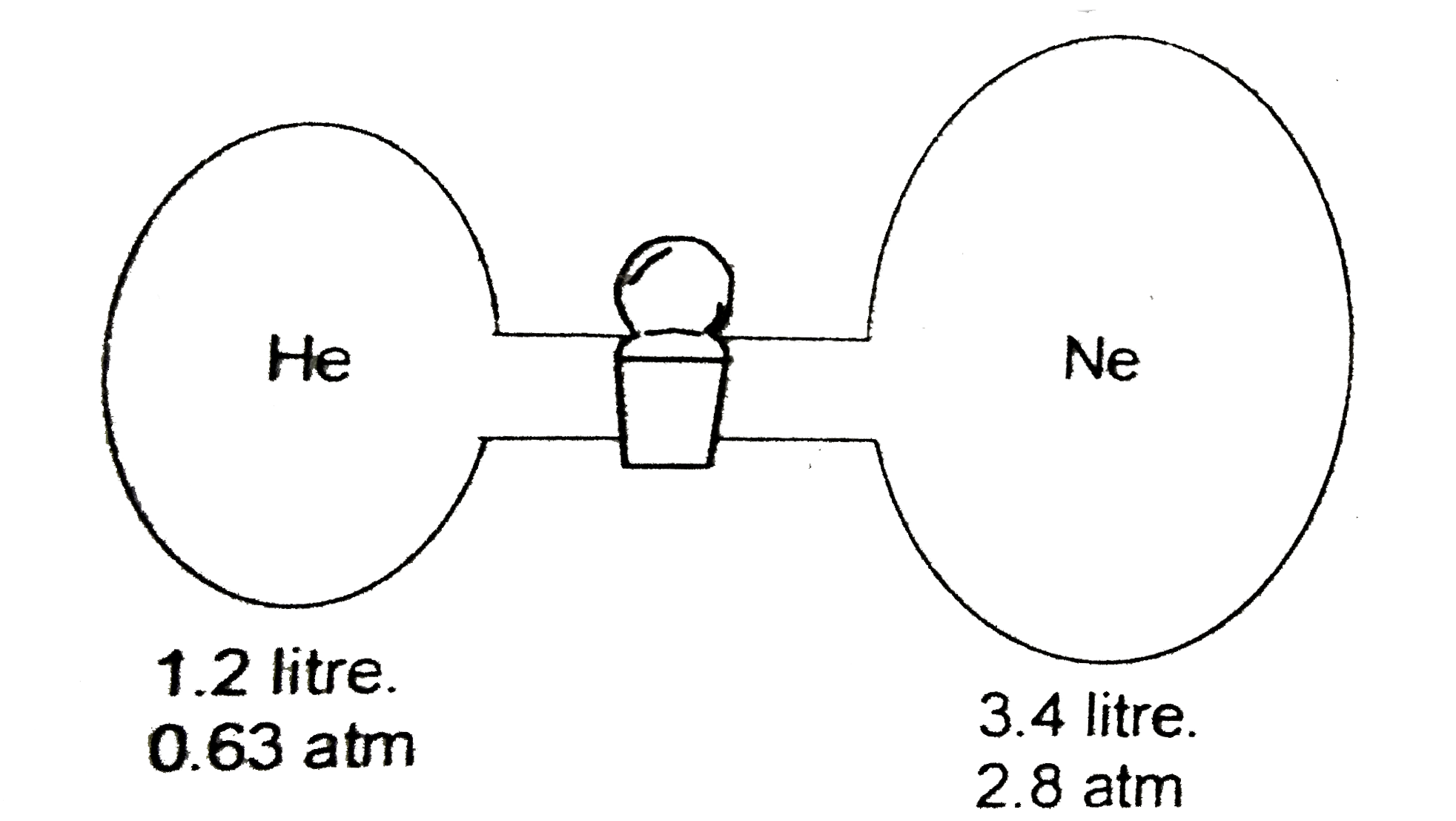
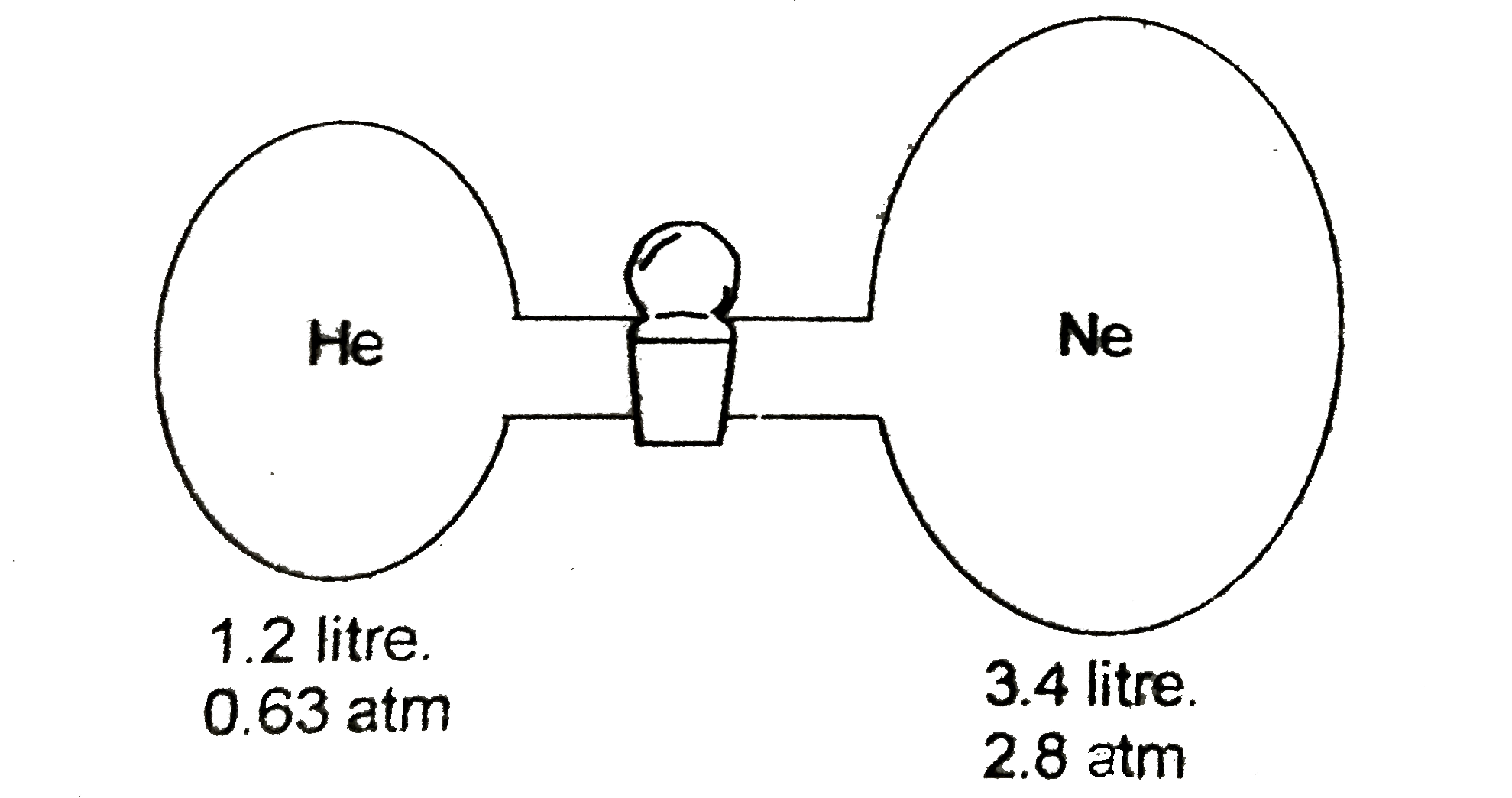
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following apparatus. Calculate the partical pressure of H...
Text Solution
|
- The density of a gas at 27^(@)C and 760 mm pressure is 24. Calculate t...
Text Solution
|
- Calculate the temperature at which rms velocity of a gas is one third ...
Text Solution
|
- Calculate the temperature at which rms velocity of a gas is half its v...
Text Solution
|
- Consider the following apparatus. Calculate the partical pressure of H...
Text Solution
|
- What will happen during the ventricular systole? a. Bicuspid and tri...
Text Solution
|
- The density of O(2) is 16 at NTP. At what temperature its density will...
Text Solution
|
- The density of O(2) is 16 at STP. At what temperature (in .^@C) its de...
Text Solution
|
- The density of O(2) is 16 at STP. At what temperature (in .^@C) its de...
Text Solution
|