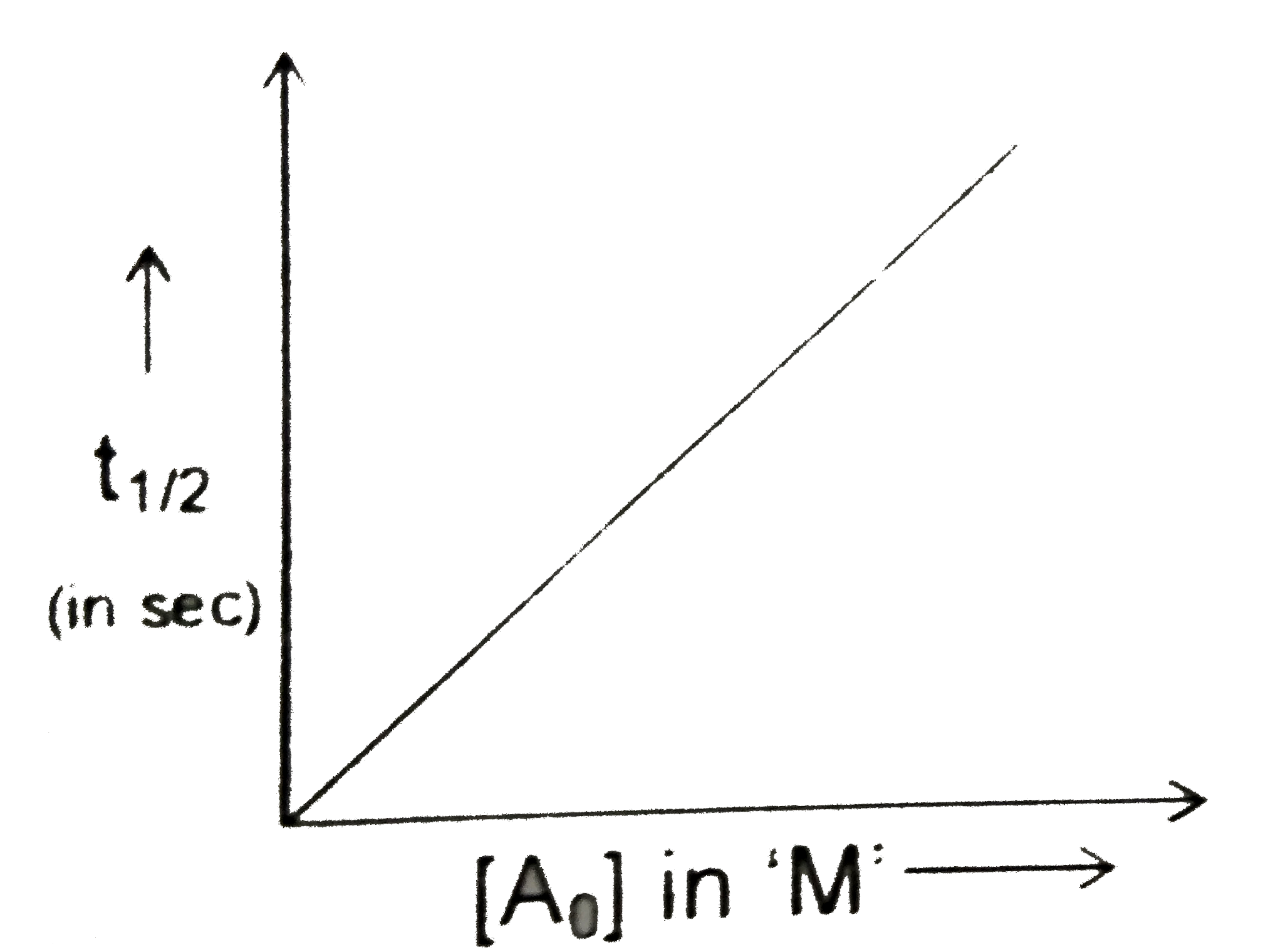
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What is the unit of rate constant of the reaction for which the above ...
Text Solution
|
- For which of the following reactions, the units of rate constant and r...
Text Solution
|
- Rate of reaction is given by the eqution : Rate = k[A]^(2)[B] . What a...
Text Solution
|
- Rate of reaction is given by the equation Rate = k[A]^(2)[B] What ...
Text Solution
|
- Rate of a reaction is given by the equation : Rate =k[A]^(2)[B] What a...
Text Solution
|
- From the following graph, identify order of reaction and mention the u...
Text Solution
|
- यदि वेग स्थिरांक की इकाई, अभिक्रिया के वेग के समान है तो अभिक्रिया की ...
Text Solution
|
- Unit of rate constant of a reaction is same as that of its rate. What ...
Text Solution
|
- the units for the rate constant and the rate of reaction are same for ...
Text Solution
|