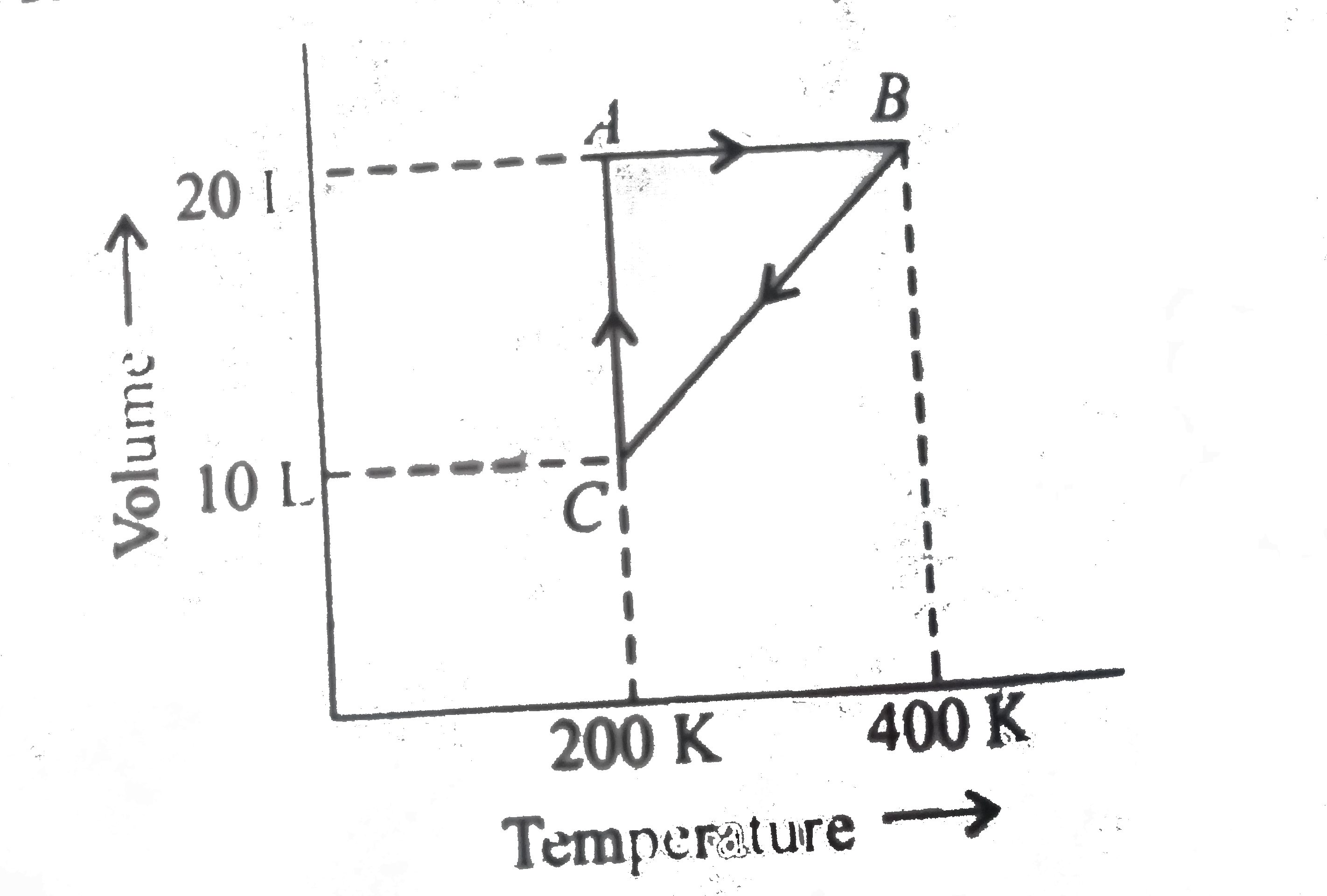
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The pressure at C is
Text Solution
|
- The pressure at C is
Text Solution
|
- Pressure of 0.210 bat at 37^(@)C. The vapour pressure of pure liquid (...
Text Solution
|
- 400 c c volume of gas having gamma=5/2 is suddenly compressed to 100 c...
Text Solution
|
- यूरिया के 0.6% जलीय घोल का 25^(@)C पर वाष्पदाब ज्ञात कीजिए. 25^(@)C ...
Text Solution
|
- If osmotic pressure of a solution is 2 atm at0^(@)C , then at 546K , ...
Text Solution
|
- किस ताप पर गैस का दाब 0.3 मीटर (पारा ) होगा | यदि 0^(@)Cपर दाब 0.273 ...
Text Solution
|
- The pressure of a gas at -73^@C is 60 cmHg.What will be its pressure a...
Text Solution
|
- 0^@C ताप पर बैरोमीटर द्वारा मापा गया दाब 760 mm है। 100^@C ताप पर दाब ...
Text Solution
|