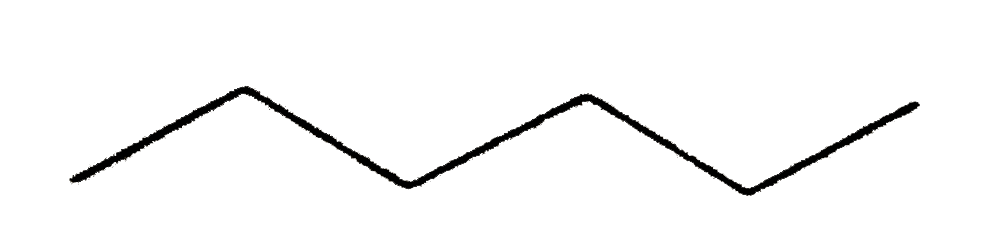
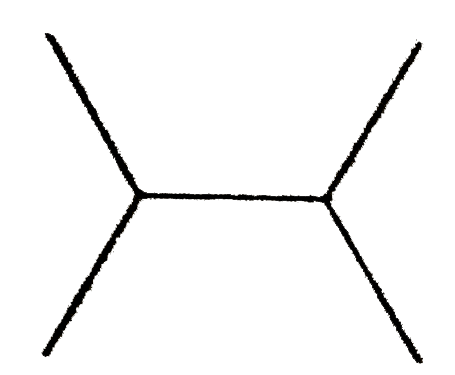
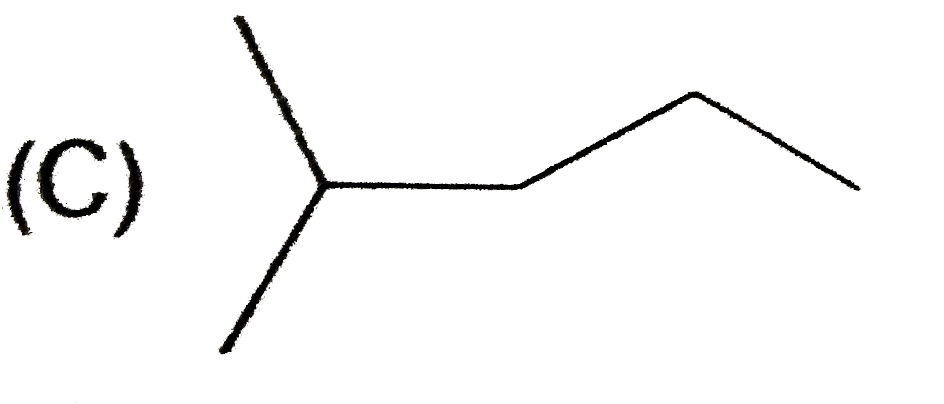
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- CH(3)CH(2)CH(2)Cl+CH(3)underset(Cl)underset(|)(C)HCH(3)underset("dry e...
Text Solution
|
- Which of the following are constitutional isomers? (i). CH(3)underset(...
Text Solution
|
- Write the structures of monomers of the following polymers (a) [-CH(...
Text Solution
|
- Ph-overset(Cl)overset(|)underset(Cl)underset(|)(C)-CH(3)overset(3NaNH(...
Text Solution
|
- Cl-CH(2)-underset(CH(3))underset(|)overset(CH(3))overset(|)C-CH(2)-CH(...
Text Solution
|
- predict the product of the following reaction underset(CH(3)-underse...
Text Solution
|
- Write the products of the following reaction: (i). CH(3)-underset(Cl...
Text Solution
|
- The major product of the following reaction is : CH(3)underset(overs...
Text Solution
|
- अभिक्रिया CH(3)-underset(Cl)underset(|)CHCH(2)CH(3) overset(Alc. KOH) ...
Text Solution
|