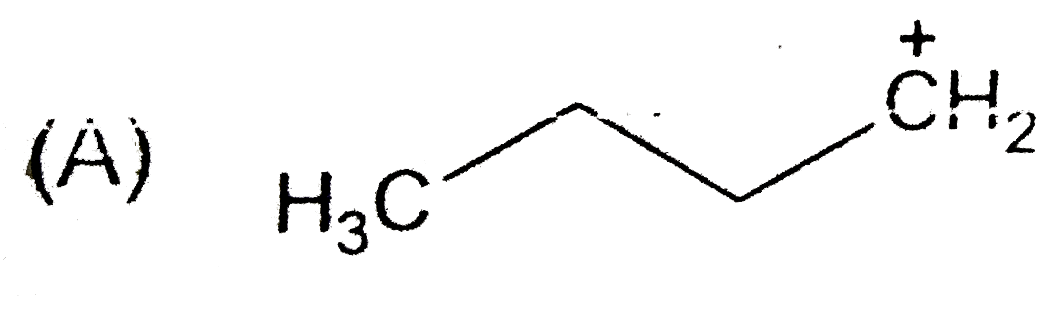
A
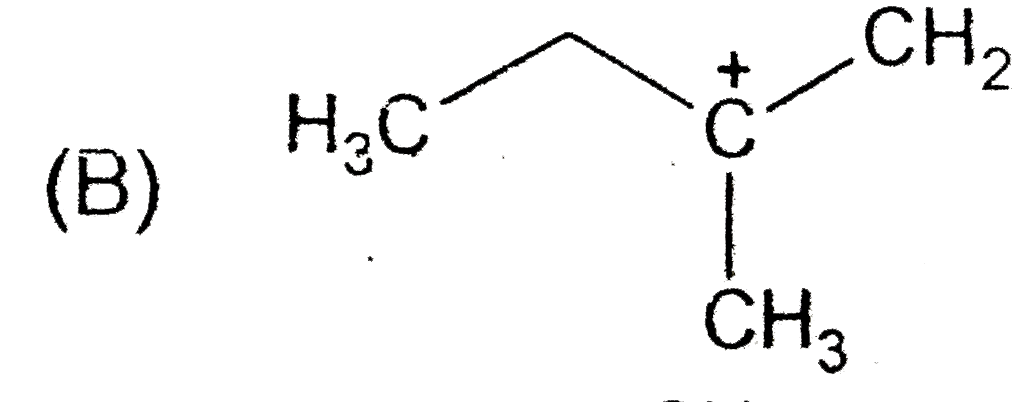
B
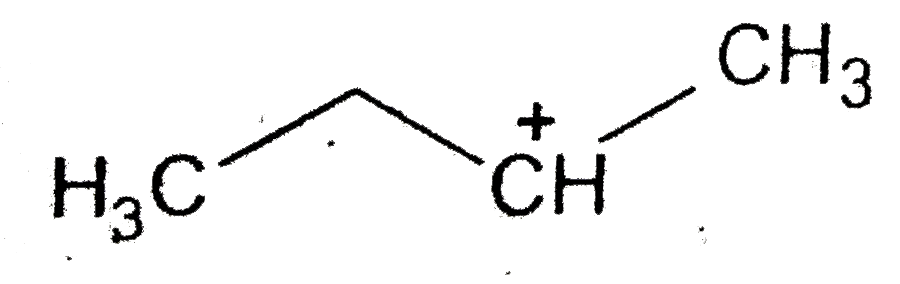
C
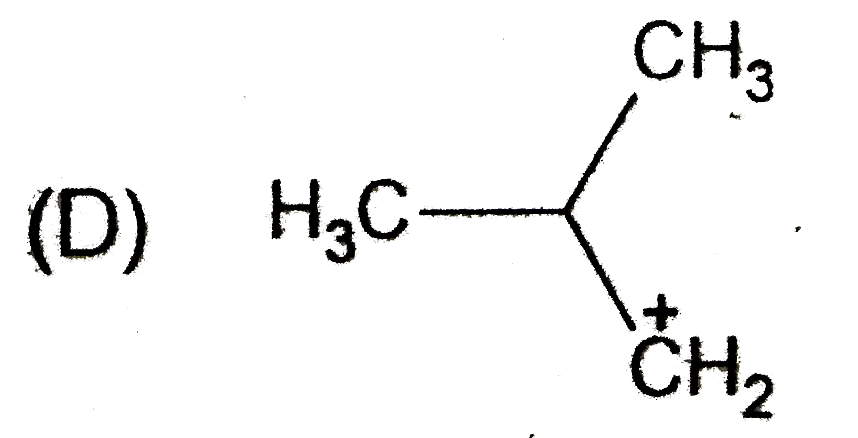
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Dehydration of 1-butanol gives 2-butane as a major product, by which o...
Text Solution
|
- Give the major and minor products of the dehydration of the given comp...
Text Solution
|
- Dehydration of 1-butanol or 2-butanol with conc. H2SO4 always gives th...
Text Solution
|
- 2-butanol on dehydration mainly gives,
Text Solution
|
- Formation of 2-butene as major on dehydration of 2-butanol is accord...
Text Solution
|
- Formation of but-2-ene as major product by dehydration of butan-2-ol i...
Text Solution
|
- …………..is obtained as the major product when 2- butanol is dehydrated.
Text Solution
|
- STATEMENT-1: 2-Methyl-2-butene is the acid catalysed dehydration produ...
Text Solution
|
- 2-ब्यूटेनॉल के निर्जलीकरण से मुख्य उत्पाद 2-ब्यूटीन निर्माण किसके अनुस...
Text Solution
|