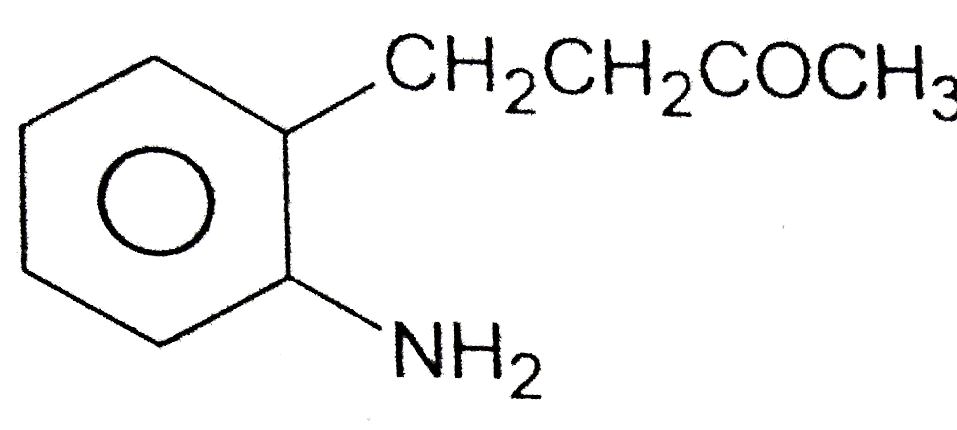
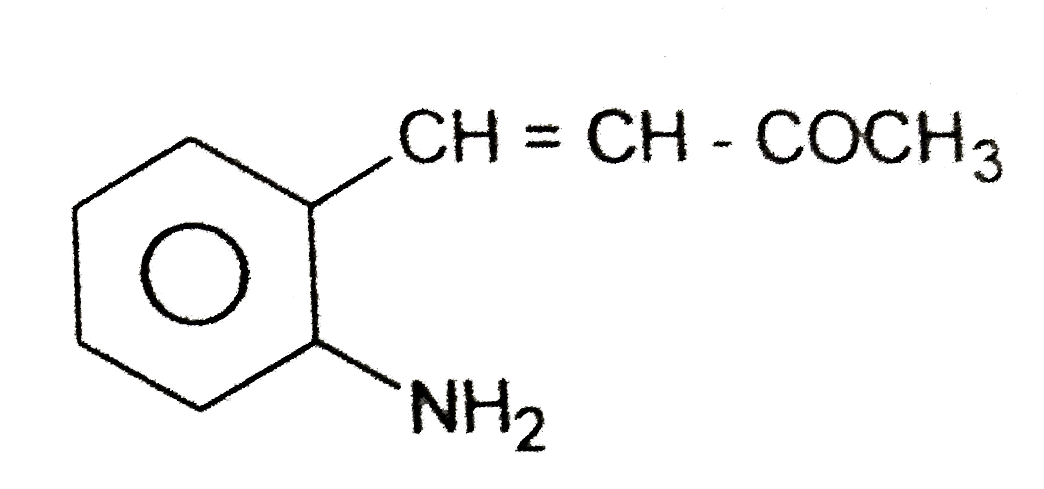
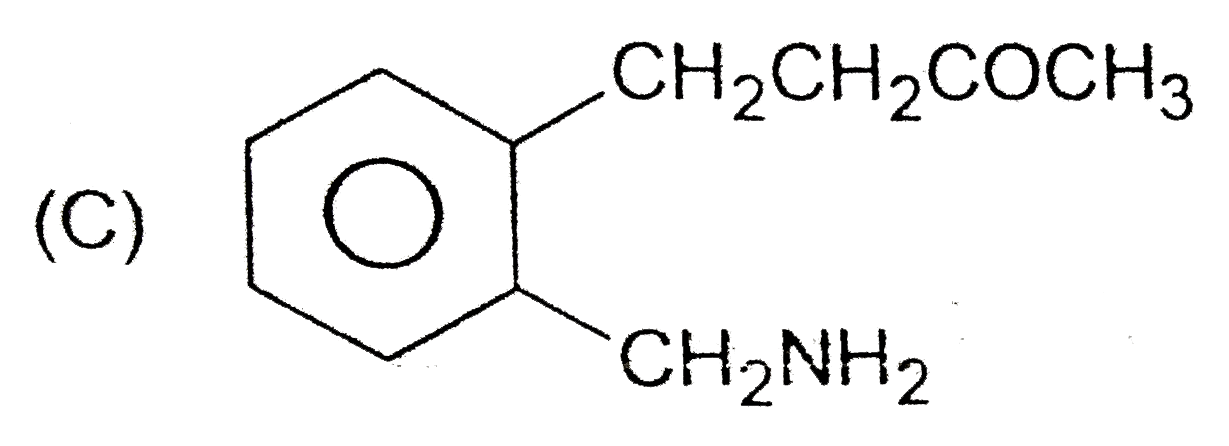
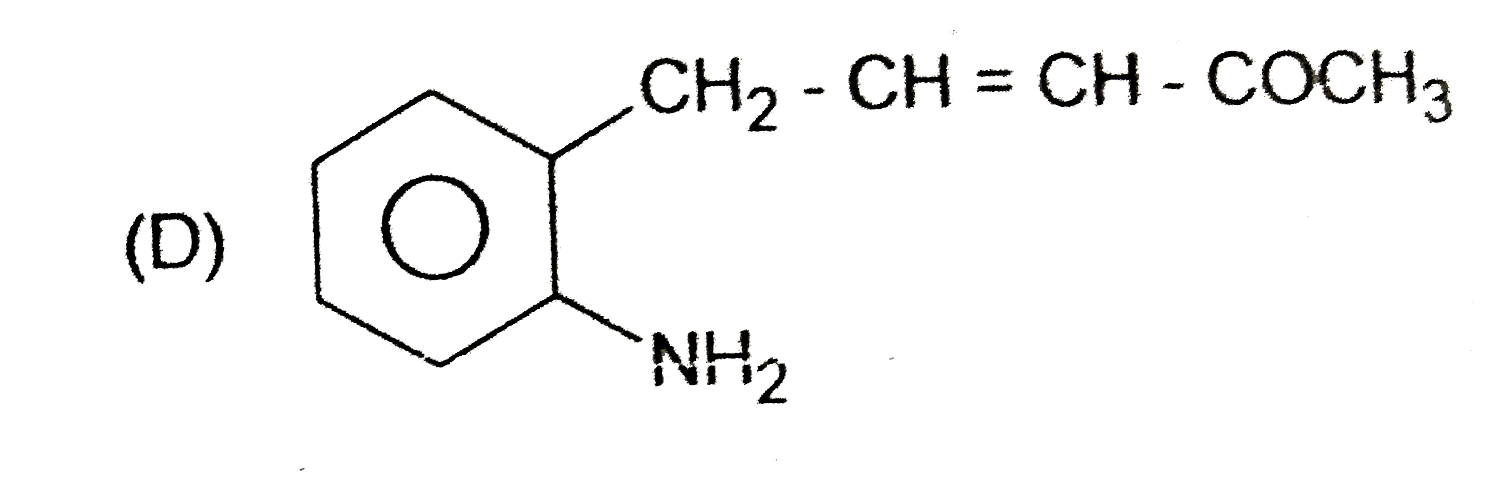
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which forms aromatic compound on heating?
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- एक रएरोमेटिक योगिक 'A' जलीय अमोनिया के साथ गर्म करने पर योगिक 'B' बनात...
Text Solution
|
- पराक्युलेट प्रदूषक निम्न में से कौन-सा एरोमैटिक यौगिक बनाता है -
Text Solution
|
- एक एरोमैटिक योगिक फॉर्मेल्डिहाइड (HCHO) के साथ क्रिया करके ताप बहुलक ब...
Text Solution
|
- Inorganic compound, which, on heating, forms organic compounds
Text Solution
|
- An aromatic compound (A) on treatment with aqueous ammonia and heating...
Text Solution
|