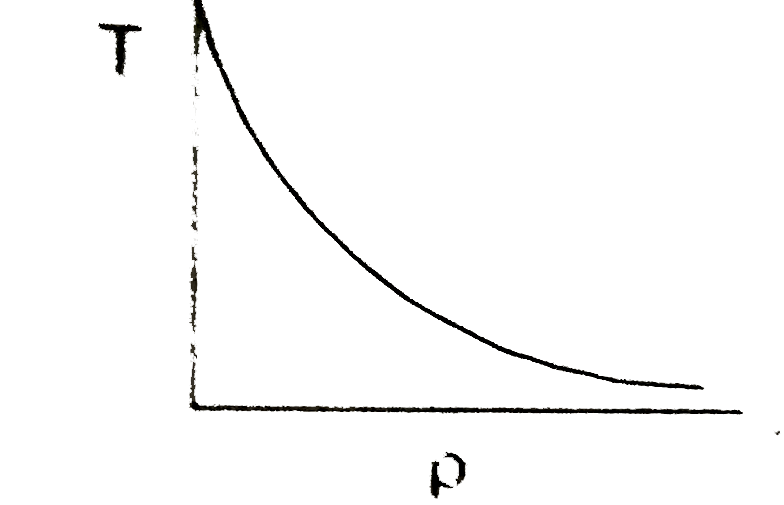
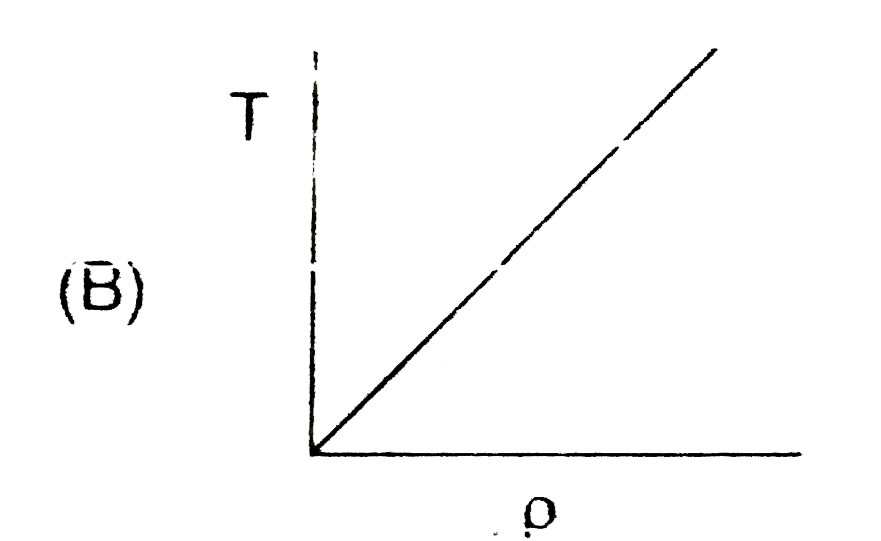
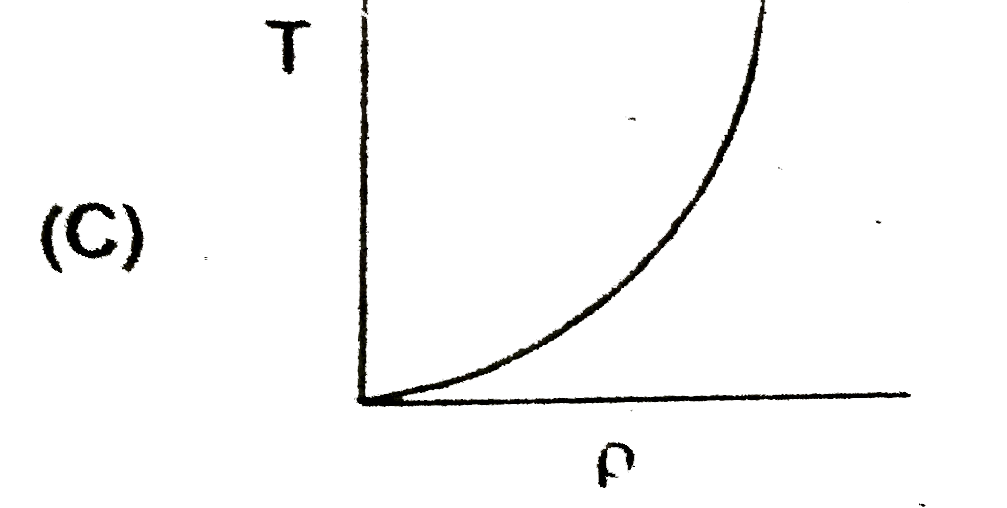
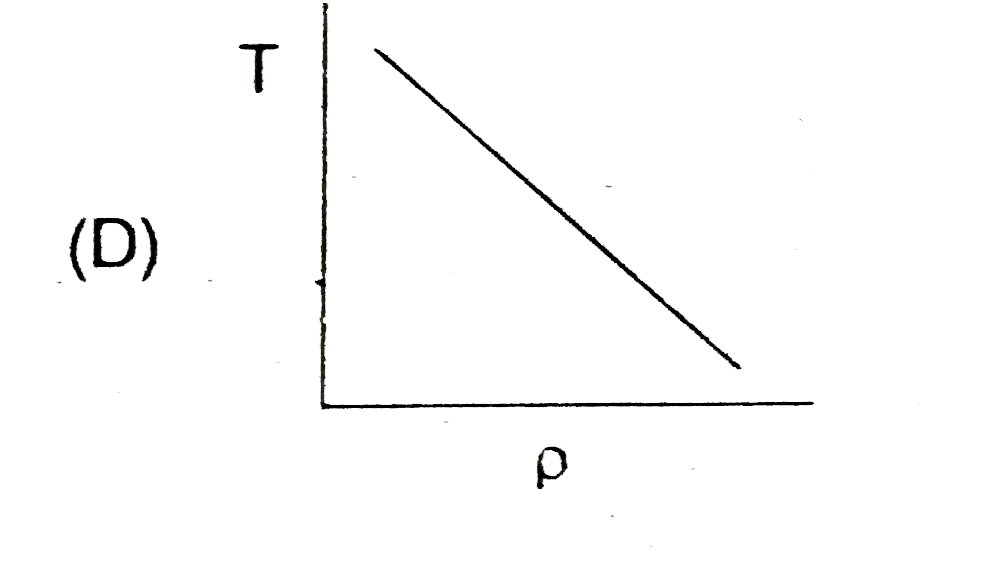
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is a graph of the relation of the density (rho)...
Text Solution
|
- During an experiment, an ideal gas is found to obey a condition (p^2)/...
Text Solution
|
- At a constant pressure, of the following graphs that one which represe...
Text Solution
|
- At a constant temperature, what is the relation between pressure P and...
Text Solution
|
- An ideal gas expands following a relation (P^(2))/(rho)= constant, whe...
Text Solution
|
- In the process PV = constant, pressure (P) versus density (rho) graph ...
Text Solution
|
- For a ideal monoatomic gas match the following graphs for constant mas...
Text Solution
|
- During an experiment, an ideal gas is found to obey a condition (p^2)/...
Text Solution
|
- Which of the following shown the correct relationship between the pres...
Text Solution
|