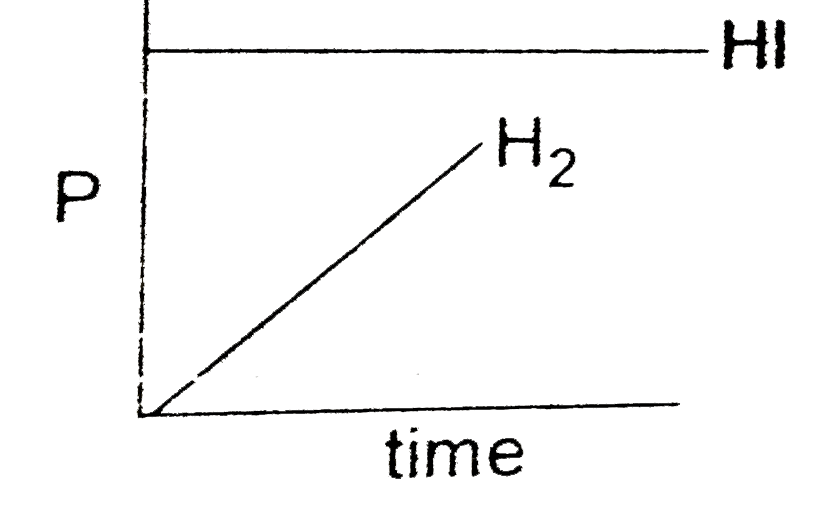
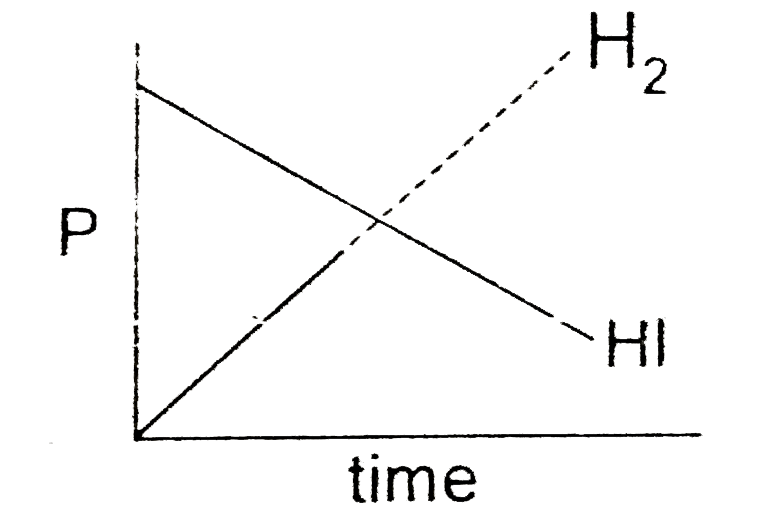
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following plot of pressure (P) Vs time satisfy decomposit...
Text Solution
|
- Based upon Boyle's law, draw the plot of P vs V and also PV vs P.
Text Solution
|
- In the plot of Z (compressibility factor) vs P,Z attains a value of un...
Text Solution
|
- In the following sketch three constant pressure lines are plotted on T...
Text Solution
|
- For 1 mole of an ideal gas, a graph of pressure vs volume is plotted a...
Text Solution
|
- For a first order chemical raction, which of the following describe th...
Text Solution
|
- For 1 mole of an ideal gas, a graph of pressure vs volume is plotted a...
Text Solution
|
- Consider the following first order decomposition and the accompanying ...
Text Solution
|
- In which of the following reactions the graph of concentration vs time...
Text Solution
|