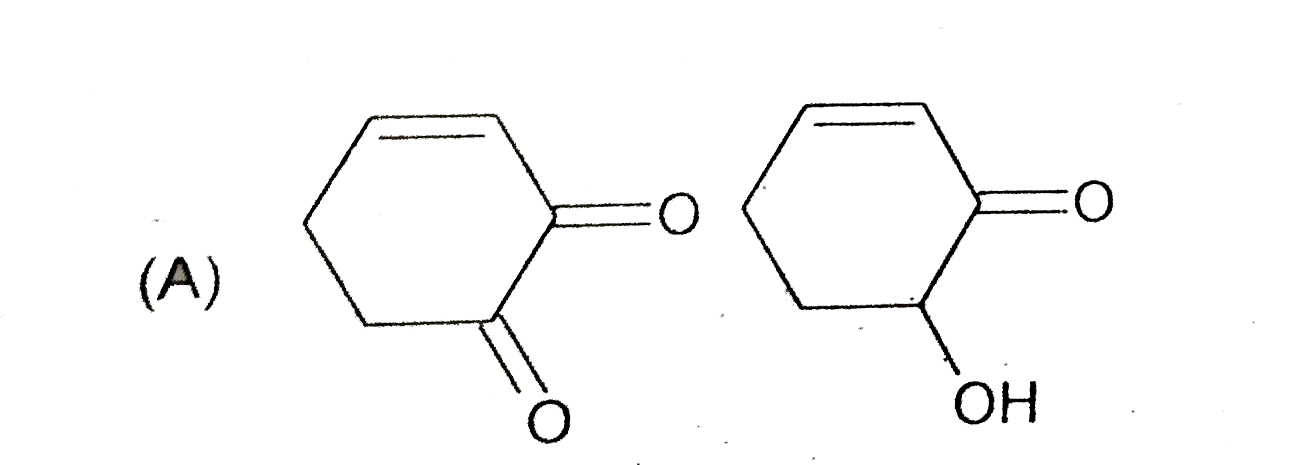
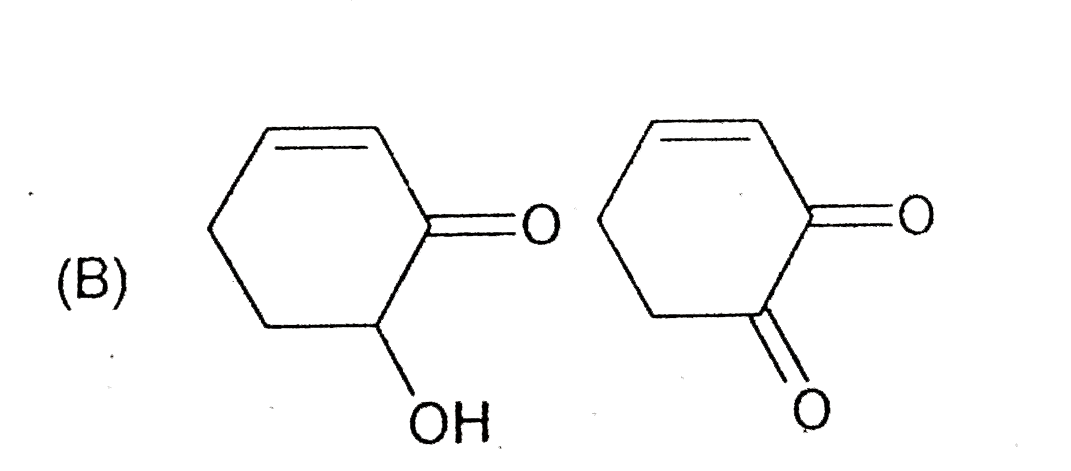
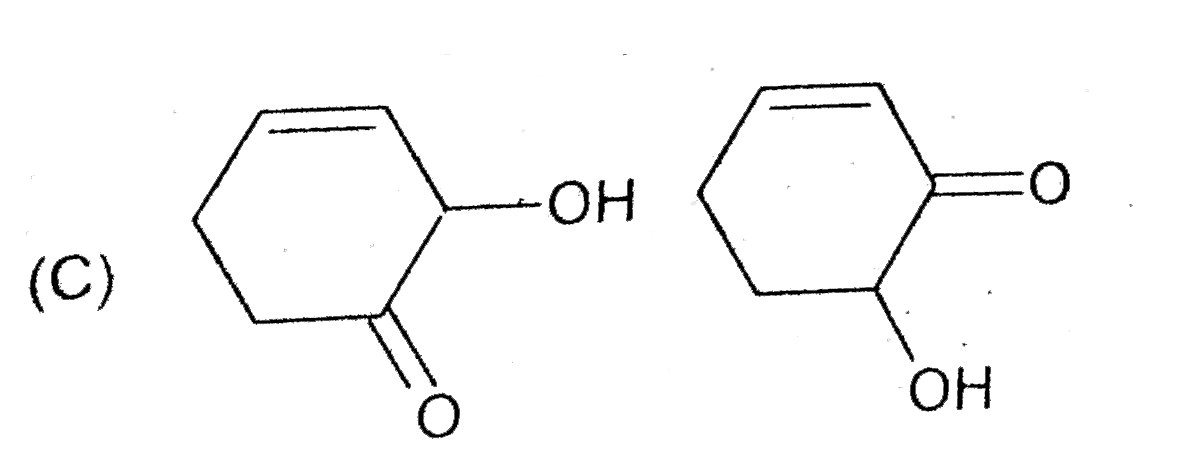
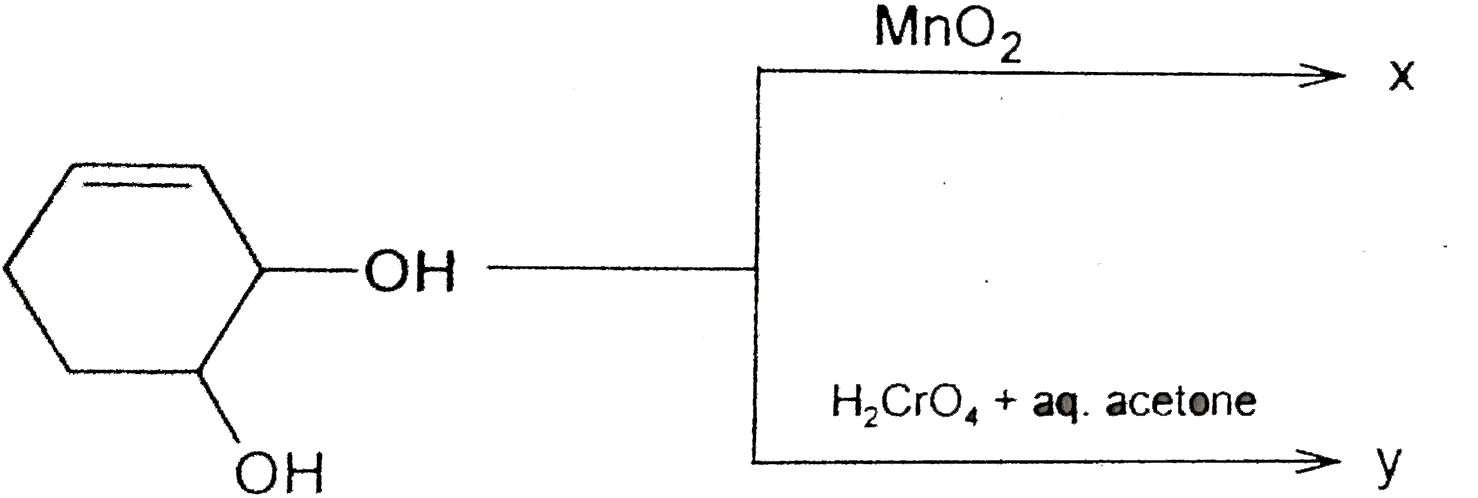A
B
C
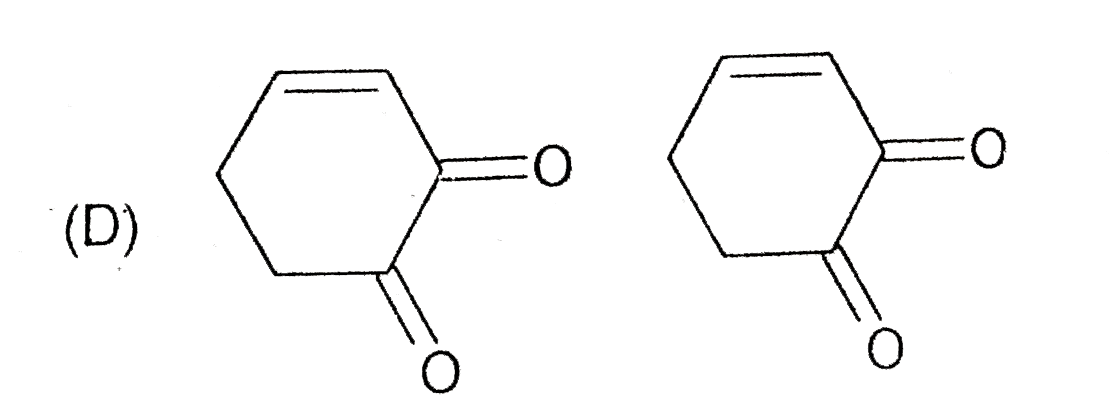
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compounds x an y are
Text Solution
|
- The compound of 'X' and 'Y' may be:
Text Solution
|
- The compound X and Y are
Text Solution
|
- Compounds [X] and [Y] are respectively:
Text Solution
|
- When compound 'X' is oxidised by acidified potassium dichromate , comp...
Text Solution
|
- Compound "X" undergoes the following sequences of reactions to form "Y...
Text Solution
|
- Unknown compound (X) on hydration by conc. H2SO4 gives (Y). The compou...
Text Solution
|
- An organic compound X on treatment with pyridinium chlorochromate in d...
Text Solution
|
- The temporary hardness of a water sample is due to compound X. Boiling...
Text Solution
|