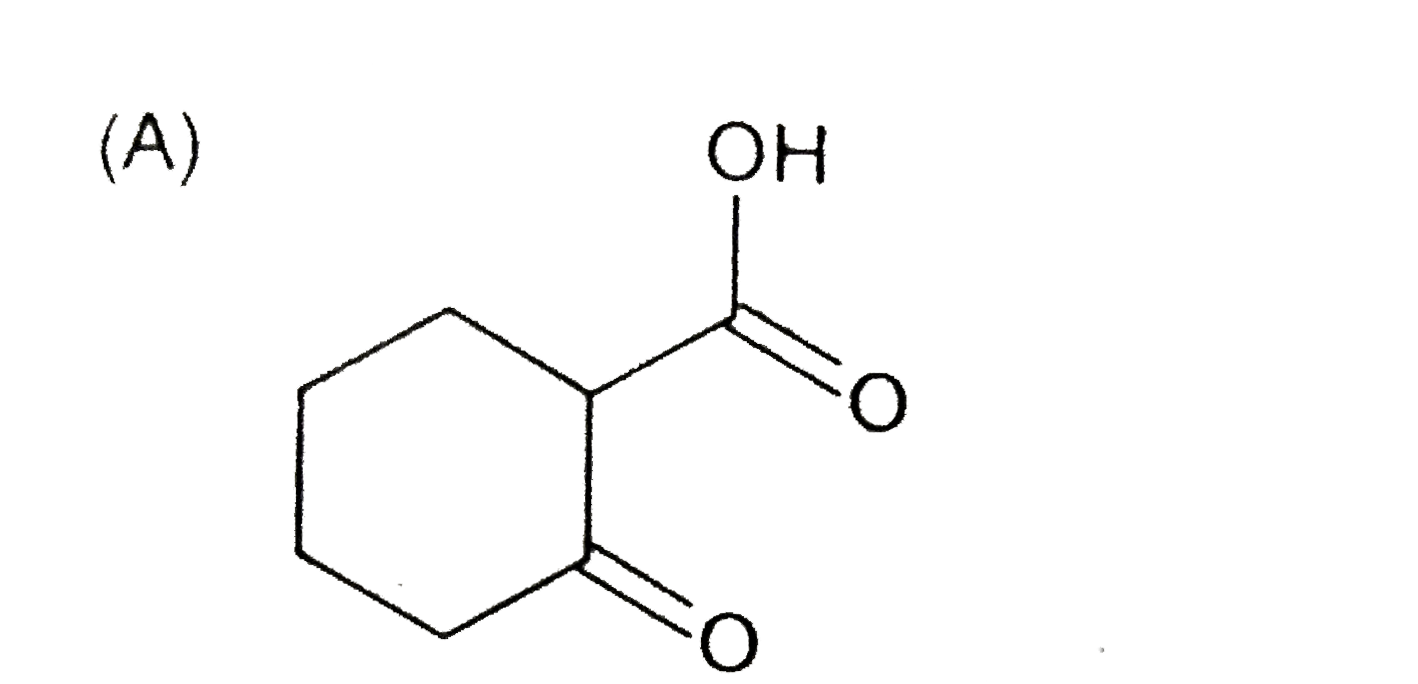
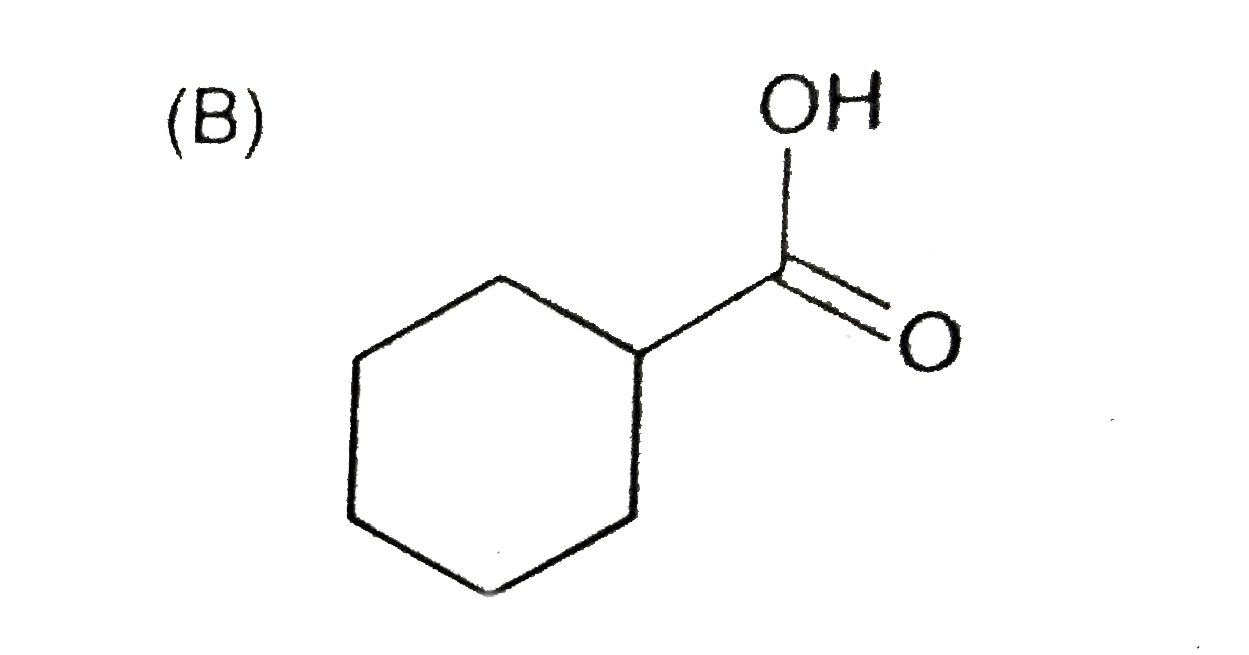
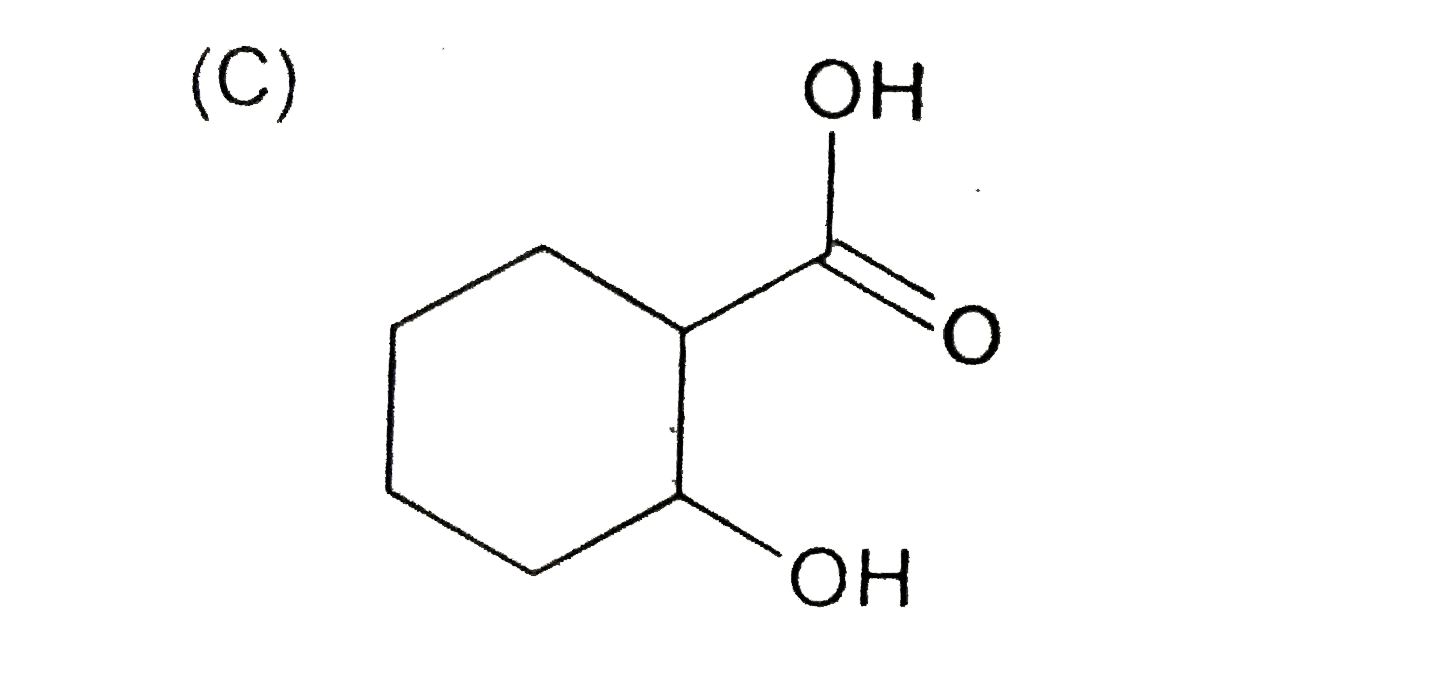
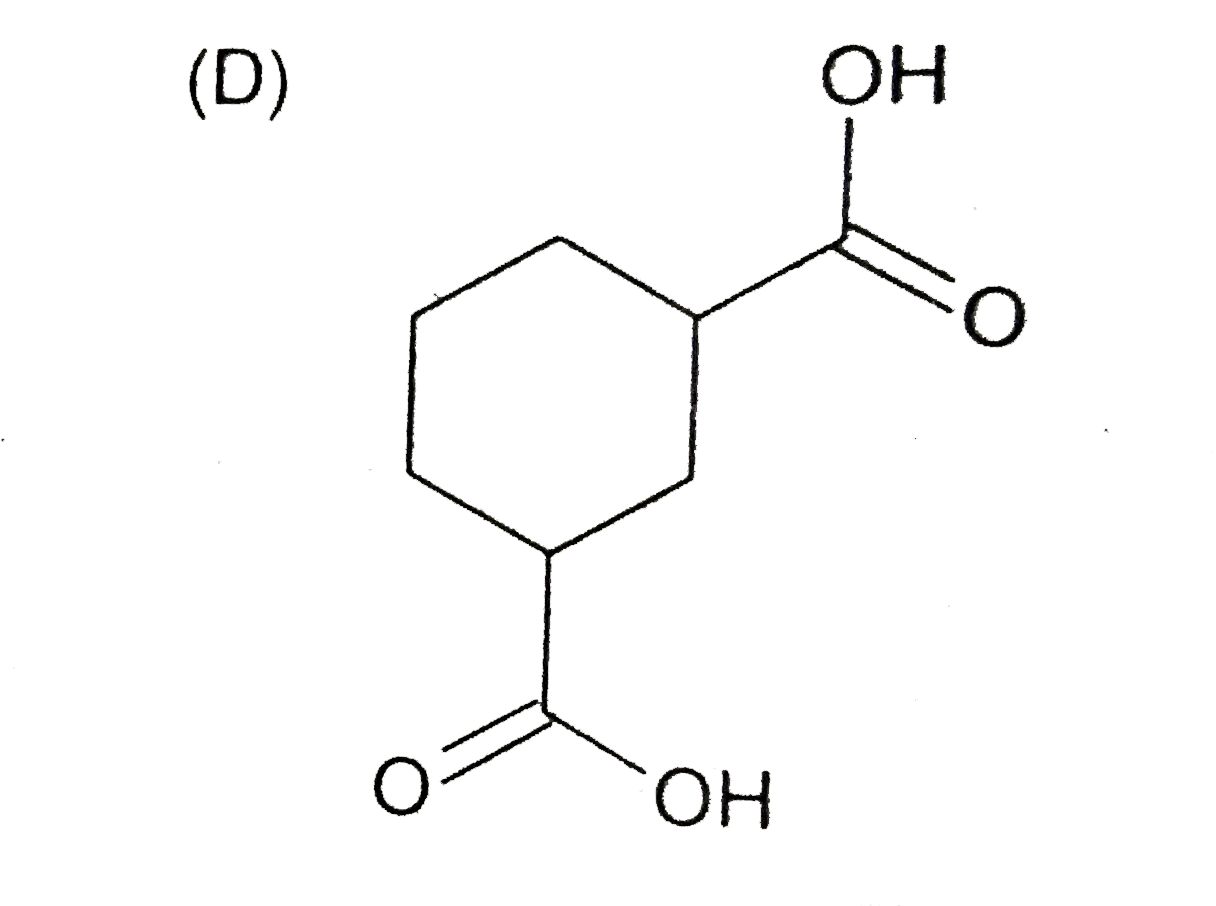
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds loses CO(2) upon heatiring to 100^(@)...
Text Solution
|
- Which of the following compounds produce CO(2) on reaction with NaHCO(...
Text Solution
|
- Which of the following compounds produce CO(2) on reaction with NaHCO(...
Text Solution
|
- Which of the following compound will not liberate CO(2) on reaction wi...
Text Solution
|
- Which of the following compound gives CO(2) on reductive ozonolysis-
Text Solution
|
- Name the alkali metal carbonate which evolves CO(2) upon heating.
Text Solution
|
- Which of the following compounds could liberate CO(2) with aqueous NaH...
Text Solution
|
- निर्जलीय Na(2)CO(3) तथा NaHCO(3) के मिश्रण को 100^(@)Cपर गर्म करन...
Text Solution
|
- Among the following which compound will react with Na(2)CO(3) solution...
Text Solution
|