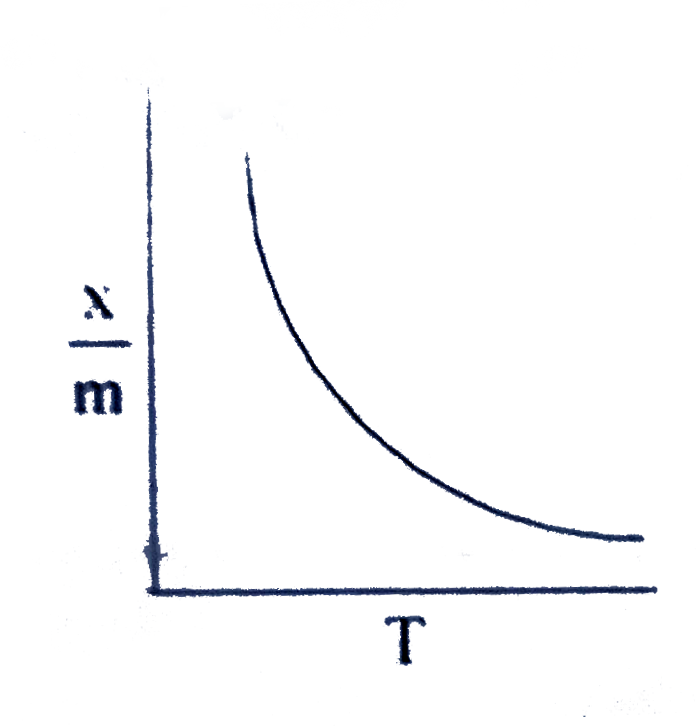
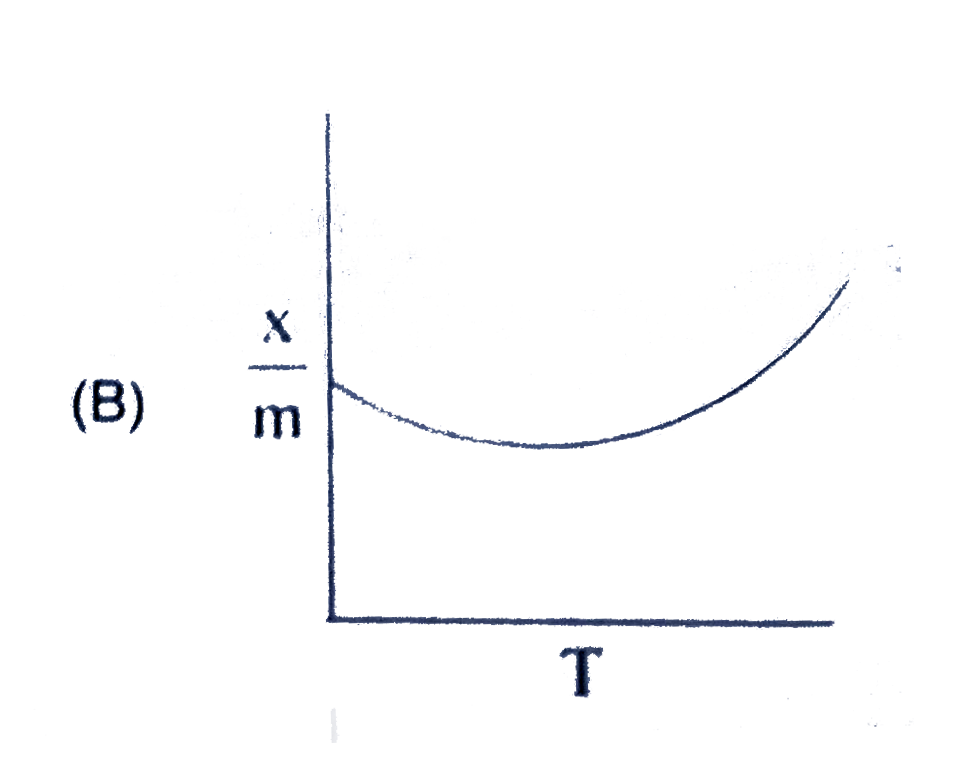
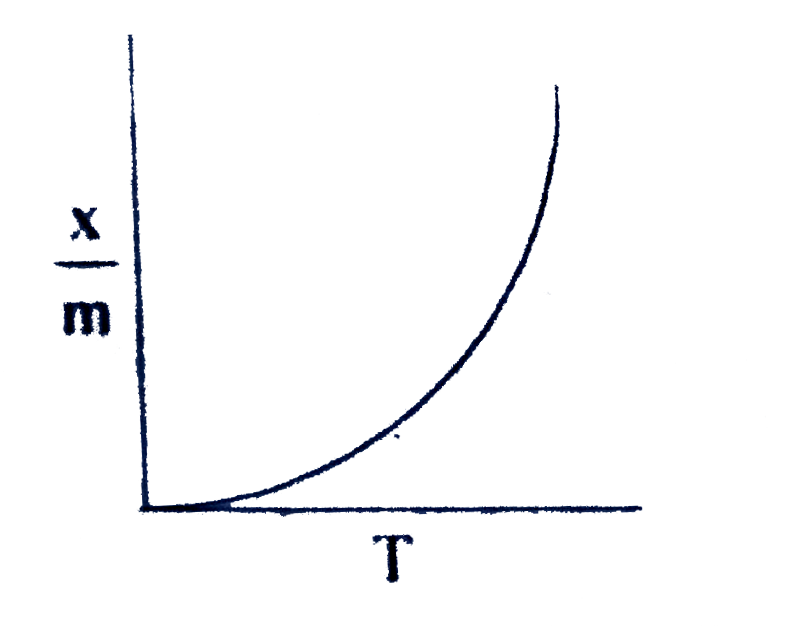
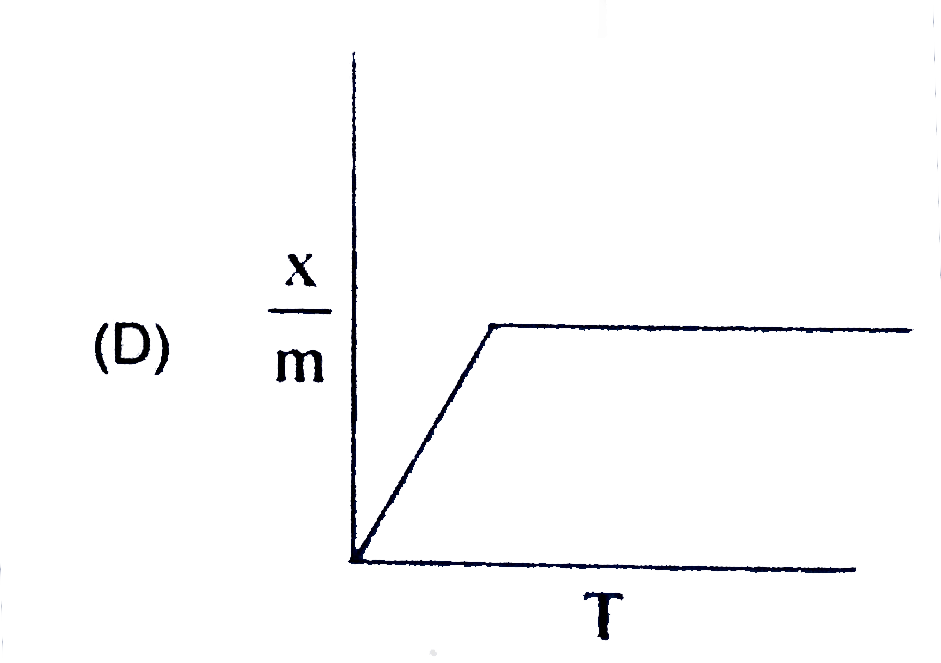
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which plot is the adsorption isobar for chemisorption?
Text Solution
|
- Which is not adsorption isobar for chemisorption?
Text Solution
|
- Which plot is the adsorptoion isobar for chemisorption where m (at con...
Text Solution
|
- Select correct adsorption isobars for chemisorption and physisorption ...
Text Solution
|
- Which plot is the adsorption isobar for chemisorption where x is...
Text Solution
|
- Of physisorption and chemisorption which type of adsorption has a high...
Text Solution
|
- Out of physisorption and chemisorption which one has lower enthalpy of...
Text Solution
|
- Which plot is the adsorption isobar for chemisorption where x is the a...
Text Solution
|
- Which of the plots is adsorption isobar for chemisorption?
Text Solution
|