A
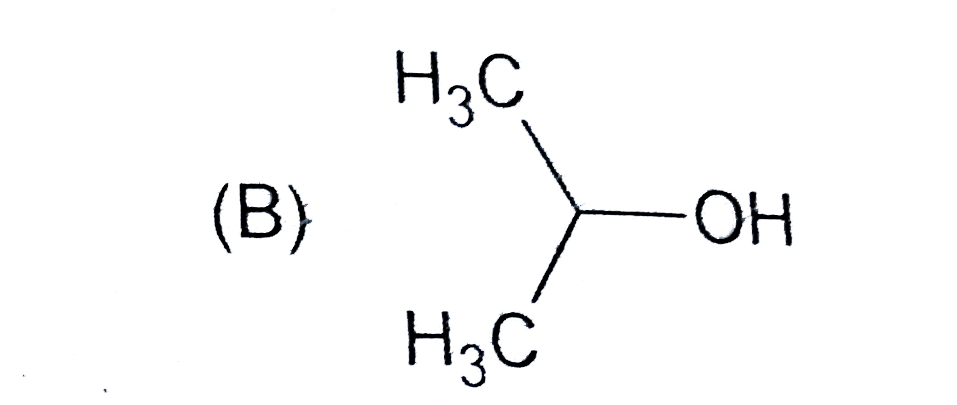
B
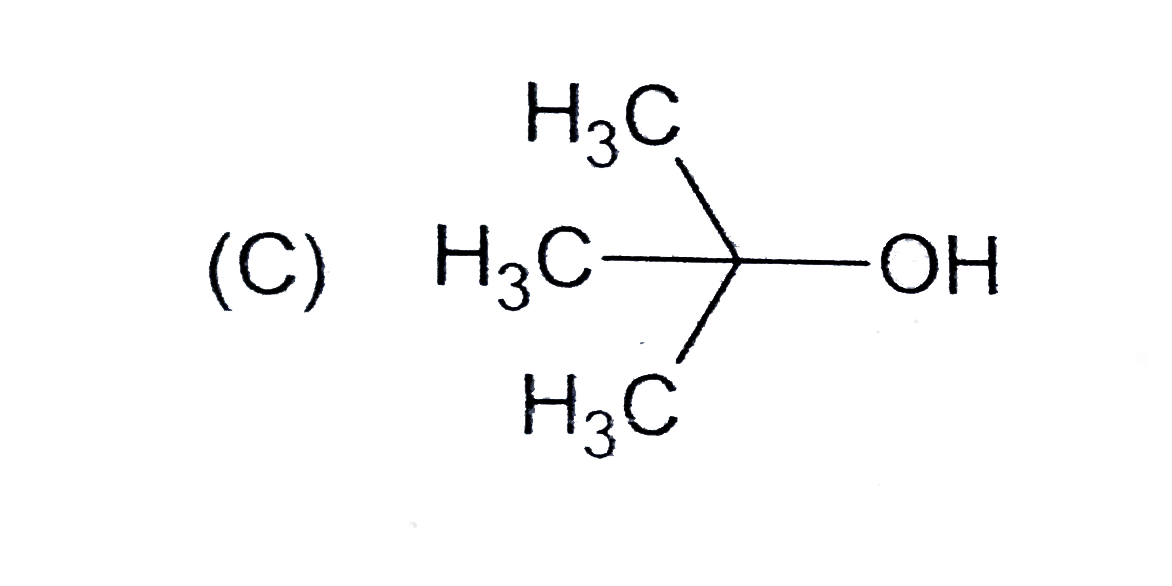
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is stronger acid than methanol?
Text Solution
|
- Asseriton: Methanol is stronger acid than water. Reason: All alcohols ...
Text Solution
|
- Explain Why : (i) Carboxylic acids are stronger acids than alcohols....
Text Solution
|
- Justify : Trichloroacetic acid is a stronger acid than dichloroacetic ...
Text Solution
|
- Which of the following is more stronger acid than Phenol ?
Text Solution
|
- The carbon-oxygen bond in phenol is slightly stronger than that in met...
Text Solution
|
- Explain: (a) Acetic Acid is a stronger acid than ethyl alcohol (b) T...
Text Solution
|
- Which of the following acids is stronger than benzoic acid (K(a)=6.3xx...
Text Solution
|
- The carbon-oxygen bond is phenol is slightly stronger than that in met...
Text Solution
|