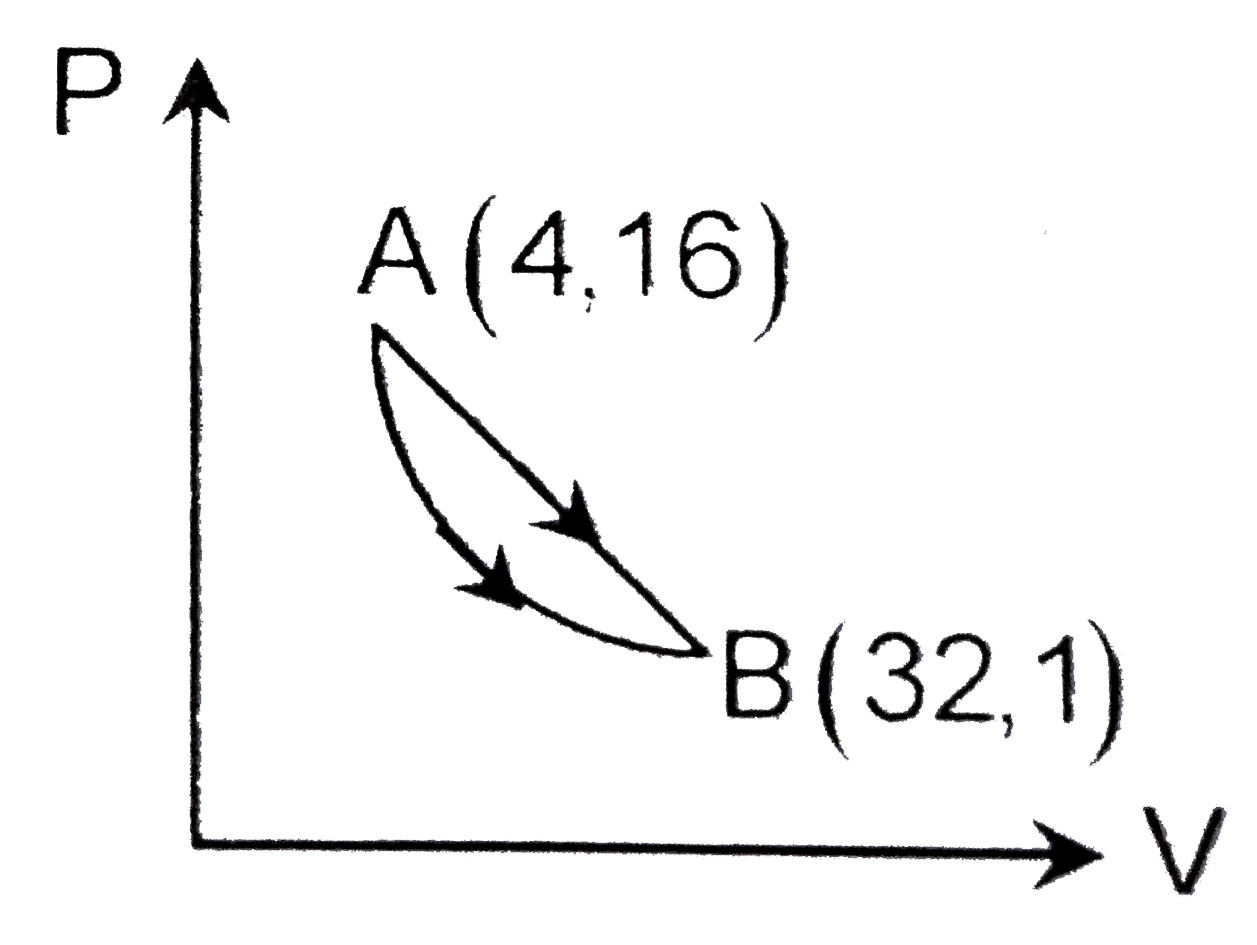
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An ideal mono atomic gas is taken through the cyclic process shown in ...
Text Solution
|
- One mole of an ideal mono-atomic gas is taken round cyclic process ABC...
Text Solution
|
- A mono atomic gas initially at 27^(@)C is compressed adiabatically to ...
Text Solution
|
- The efficiency of an ideal gas with adiabatic exponent 'gamma' for the...
Text Solution
|
- In an adiabatic process, the work involed during expansion or compr...
Text Solution
|
- A sample of ideal gas is taken through the cyclic process shown in the...
Text Solution
|
- The efficiency of an ideal gas with adiabatic exponent 'gamma' for the...
Text Solution
|
- In adiabatic process, the work involved during expansion or compressio...
Text Solution
|
- (a) A sample of 1.0 mol of a monoatomic ideal gas is taken through a ...
Text Solution
|