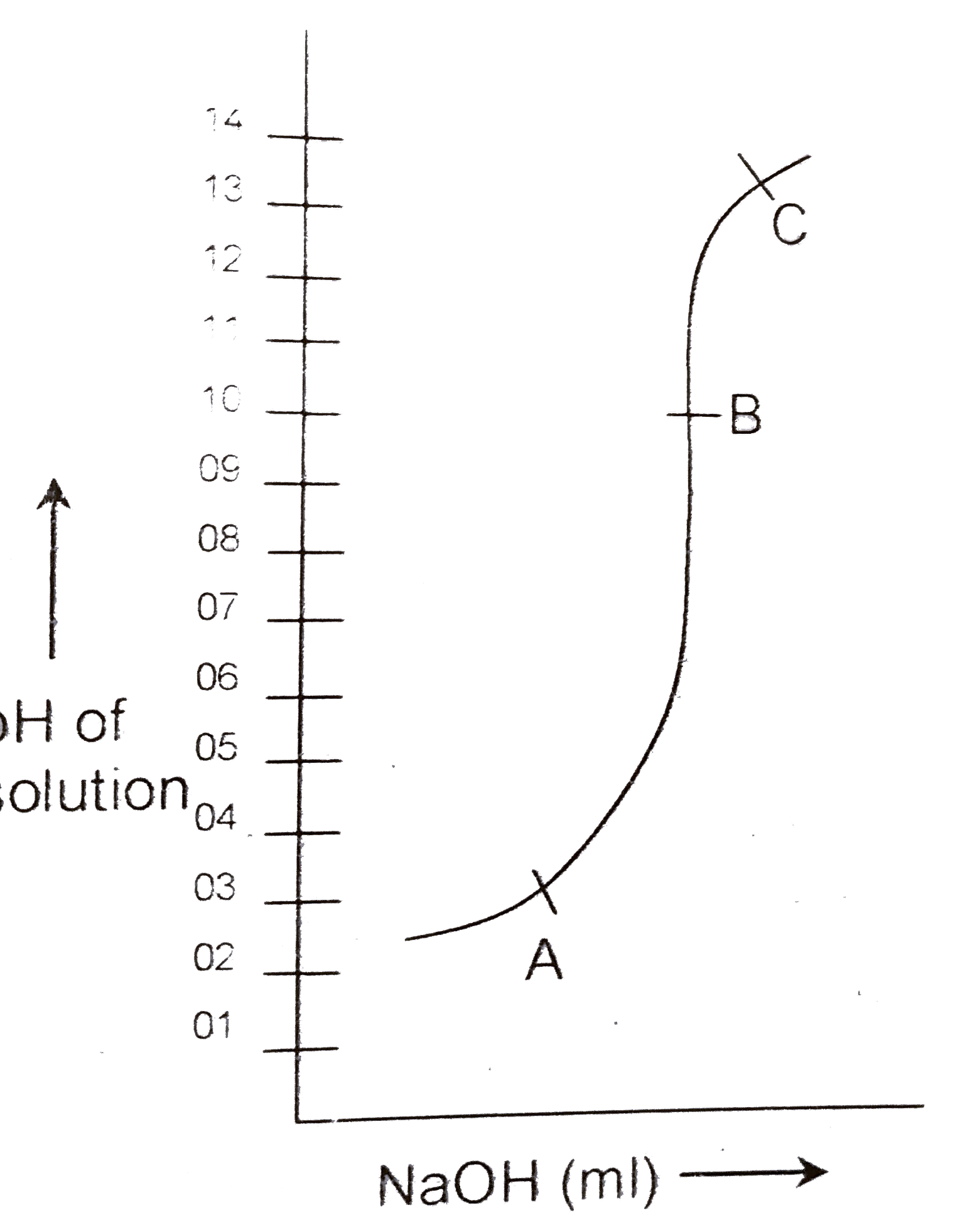
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A 0.1 M solution of a weak acid HA is titrated by NaOH slowly. The ti...
Text Solution
|
- A weak acid (HA) is titrated with N//100 NaOH . What will be the pH wh...
Text Solution
|
- Acetic acid is titrated with NaOH solution. Which of the following sta...
Text Solution
|
- A sample of 100 mL of a solution of a weak monoprotic acid of unknown ...
Text Solution
|
- Calculate [H^(+)] at equivalent point between titration of 0.1 M, 25 m...
Text Solution
|
- Which of the following curves corresponds to the titration of a weak b...
Text Solution
|
- A 25.0 mL of 0.1 M weak acid HA is titrated with 0.1 M NaOH to the eq...
Text Solution
|
- 1.2 of a monprotic acid HA, is titrated with 0.222 M NaOH solution. Th...
Text Solution
|
- 1.2 of a monprotic acid HA, is titrated with 0.222 M NaOH solution. Th...
Text Solution
|