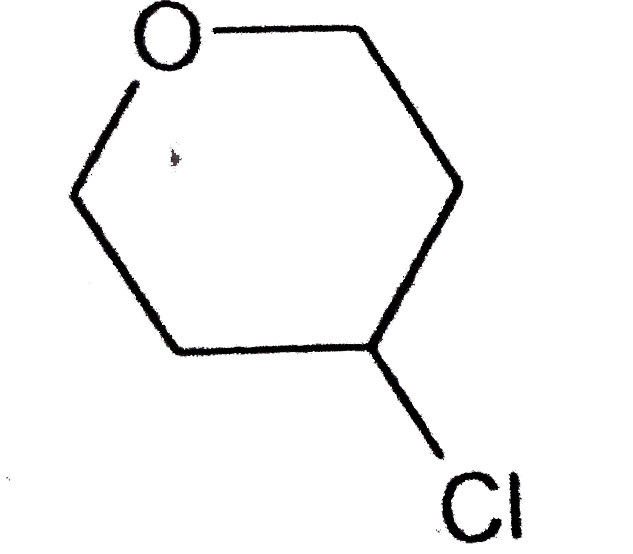
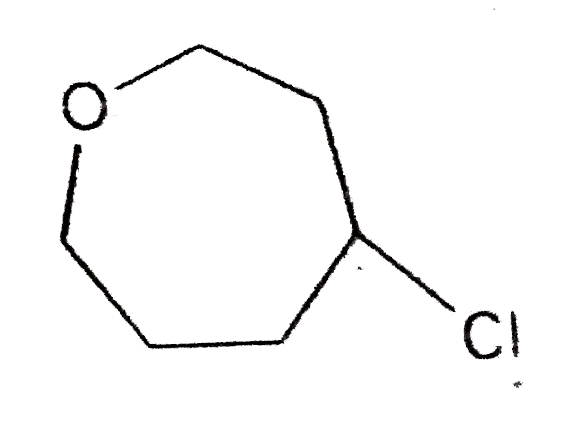
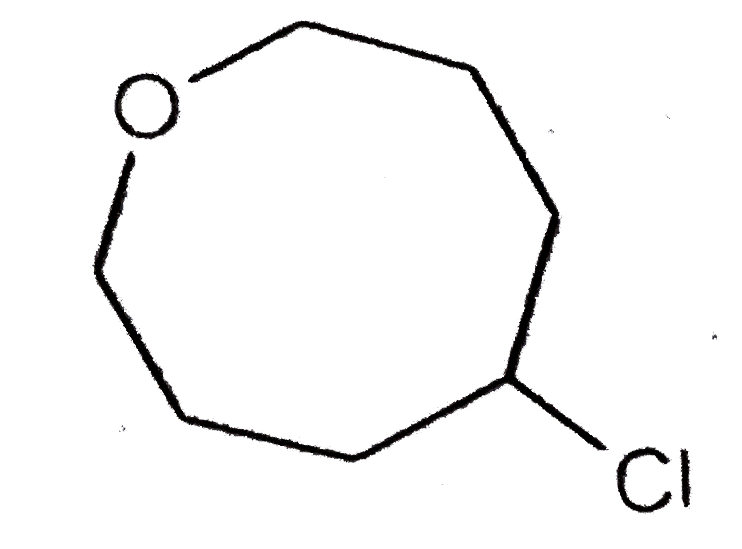
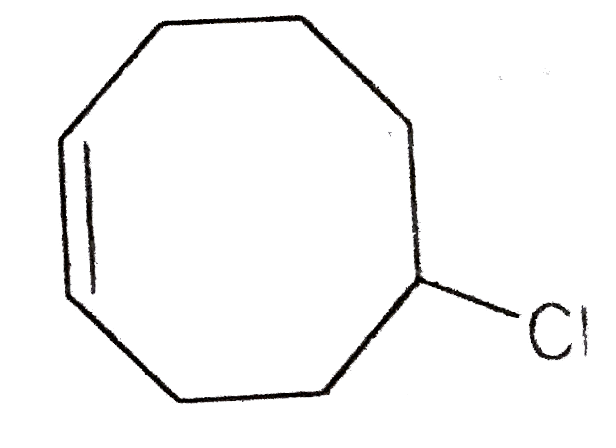
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following undergoes faster solvolysis ?
Text Solution
|
- Which of the following haloalkanes would undergo S(N^(2)) reaction fas...
Text Solution
|
- Which compound in the following pair undergoes faster SN1 reaction?
Text Solution
|
- Which one of the following chlorohydrocarbons readily undergoes solvol...
Text Solution
|
- Which one of the following chlorohydrocarbons readily undergoes solvol...
Text Solution
|
- Which of the following compounds most readily undergoes solvolysis by ...
Text Solution
|
- Which of the following compounds most readily undergoes solvolysis by ...
Text Solution
|
- Predict the compound which will undergo solvolysis (aqueous ethanol) m...
Text Solution
|
- Which one of the following alkyl bromides undergoes most rapid solvoly...
Text Solution
|