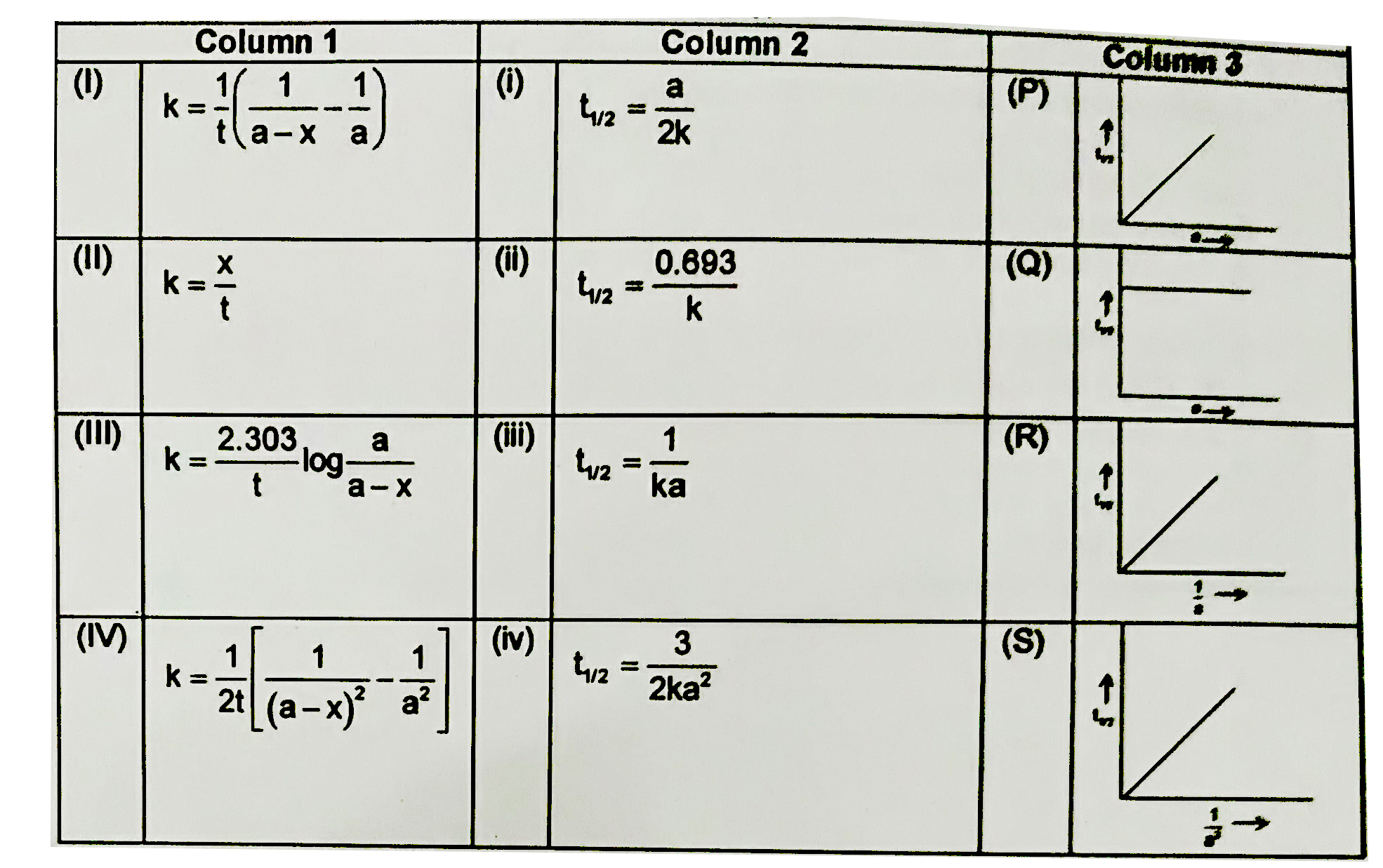
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction N(2)O(6)to2NO(2)+1/2O(2) the only correct combination...
Text Solution
|
- The compoistion of N(2)O(5) is a first order reaction represented by: ...
Text Solution
|
- Given the following reaction : P : N(2)(g)+2O(2)(g)to2NO(2)(g),DeltaH(...
Text Solution
|
- PbO(2)+O(3)rarrPbO+2O(2),BaO(2)+O(3)rarrBaO+2O(2) these reaction are...
Text Solution
|
- The reaction N(2)O(5) to 2NO(2)+I//2O(2) is of first order in N(2)O(5)...
Text Solution
|
- The reaction : N(2)O(5)( "in" (C)Cl(4) "solution") to2NO(2)("same solu...
Text Solution
|
- If equlibrium constnats of reaction, N(2)+O(2)hArr 2NO is K(1) and 1...
Text Solution
|
- अभिक्रिया N(2) +2O(2) ltimplies 2NO(2) के लिए K( c)=100 लीटर मोल^(-1) ...
Text Solution
|
- दी गई अभिक्रिया N(2)O(4)to2NO(2)+O(2) के लिए K(p) की इकाई है
Text Solution
|