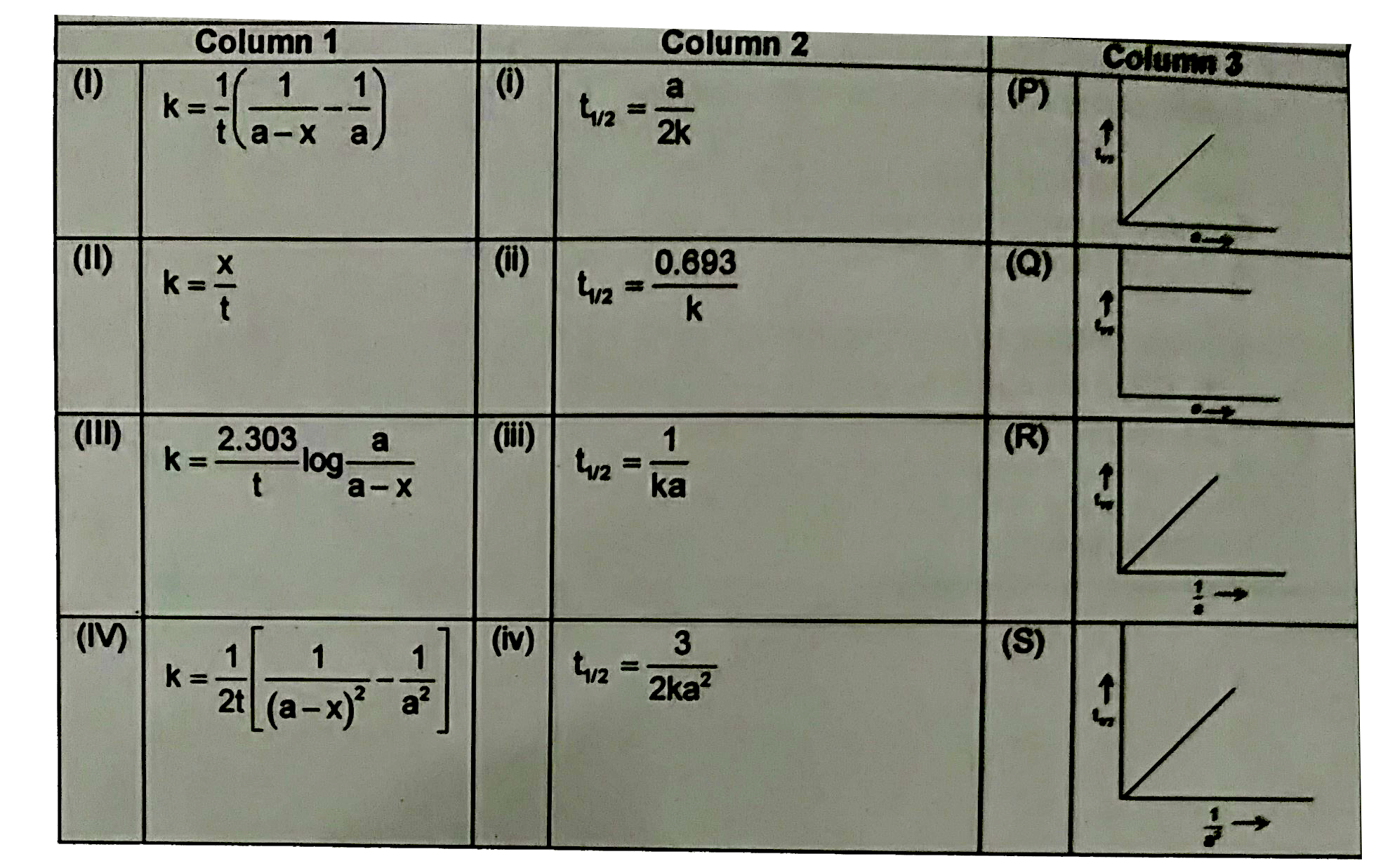
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the reaction 2AtoProduct, the rate equation is r=k[A]^(2) Which ...
Text Solution
|
- For a reaction, A+B to Products, the rate law is given by: r=k[A]^(1//...
Text Solution
|
- Rate law expression of a reaction is : Rate = k[A]^(2//3)[B] Which of ...
Text Solution
|
- यदि अभिक्रिया A + B to उत्पाद के लिए दर – नियम r= k[A]^(1//2) [B...
Text Solution
|
- For a reaction,A +B to Product : the rate law is given by r =k [A]^(1/...
Text Solution
|
- For a reaction, A+B to Products, the rate law is given by: r=k[A]^(1//...
Text Solution
|
- For a reaction , A+Brarr product, rate law is given by, r=k[A]^(1//2)x...
Text Solution
|
- For a reaction : 2A + 2B rarr products, the rate law expression is r =...
Text Solution
|
- For a reaction A + B to Product, the rate law is given by r = k[A]^(1/...
Text Solution
|