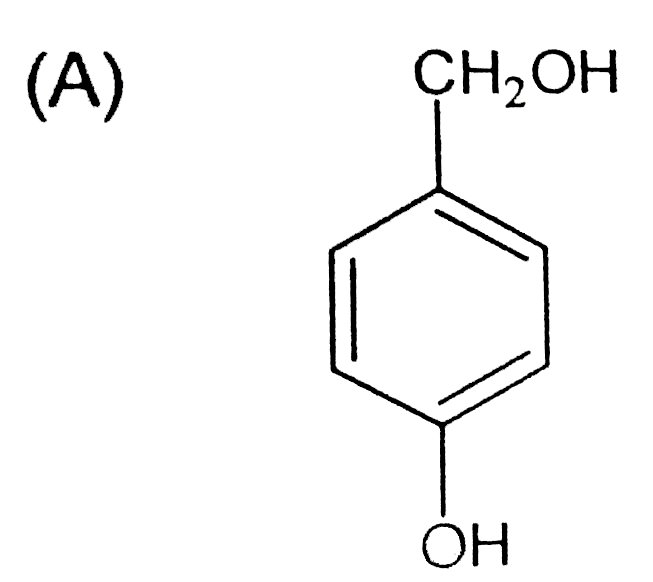
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following can give aldehyde by the oxidation with Jones r...
Text Solution
|
- Which of the following reagents cannot be used for the oxidation of 1^...
Text Solution
|
- Which of the following reagents cannot be used for the oxidation of 1^...
Text Solution
|
- The oxidation of CH(3)CH=CHCH(OH)CH(3) with Jones reagent gives
Text Solution
|
- Grignard reagent can give aldehyde with :
Text Solution
|
- Which of the following will give an aldehyde on oxidation ?
Text Solution
|
- एल्डिहाइड निम्न में से किसके द्वारा ऑक्सीकृत हो सकते हैं?
Text Solution
|
- Which of the following reagent is used to get aldehyde from alcohol by...
Text Solution
|
- প্রাইমারি অ্যালকোহলকে অ্যালডিহাইডে জারিত করার জন্য নীচের কোন্ বিকারকগু...
Text Solution
|