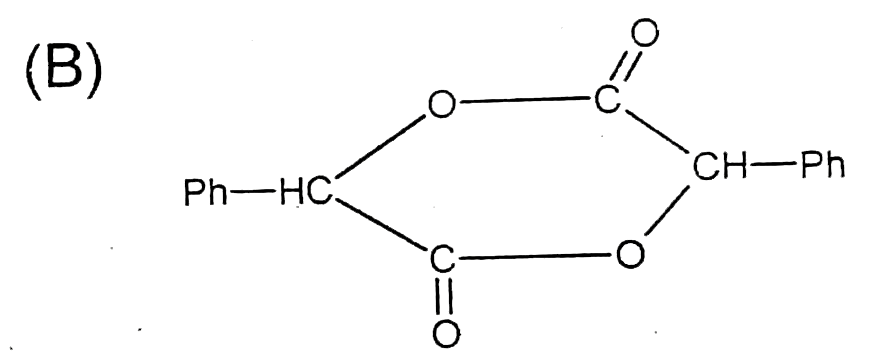
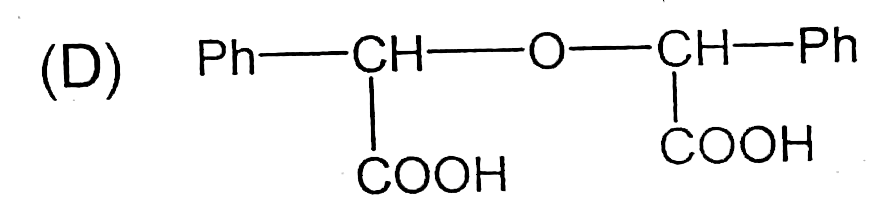A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Identify the final product in the given reaction: Ph-CH(2)COOH under...
Text Solution
|
- Identify final product in the following reaction, CH(3)underset(OH)u...
Text Solution
|
- Identify the reaction and write the IUPAC name of the product formed :...
Text Solution
|
- Identify final product In the following reaction CH(3)-underset(OH)und...
Text Solution
|
- CH(3)COOH underset(P(2)O(5))overset(Delta)toX . Identify X
Text Solution
|
- Final product of following reaction is CO(2) + (CH(3))(3) C - MgBr und...
Text Solution
|
- In the given reaction CH(3)COOH underset({:((ii)NaCN),((iii)H(2)O //H^...
Text Solution
|
- Complete the following reactions: CH(3)-overset(CH(3))overset(|)(CH)...
Text Solution
|
- CH(3)-overset(CH(3))overset("| ")underset(CH(3))underset("| ")"C "-C...
Text Solution
|