Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
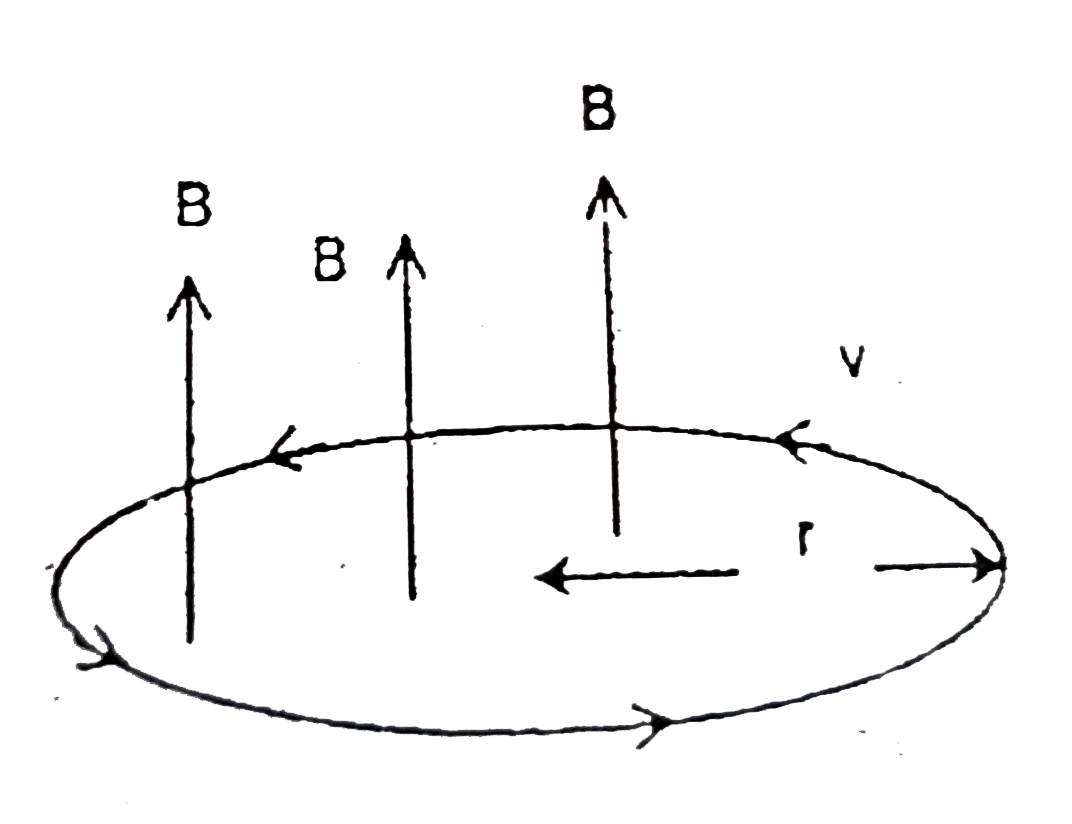
- An electron is orbiting is a circular orbit of radius r under the infl...
Text Solution
|
- An electron is orbiting is a circular orbit of radius r under the infl...
Text Solution
|
- Deduce the expression for the magnetic field induction at the centre o...
Text Solution
|
- Select the correct curve (s) : If = V Velocituy of electron in Bohr'...
Text Solution
|
- An electron revolve round the nucleous with the radius of the circular...
Text Solution
|
- The orbital speed of an electron orbiting around the nucleus in a circ...
Text Solution
|
- Select the correct curve(s) : If v = velocity of electron in Bohr’s ...
Text Solution
|
- सही वक्र (वक्रो) का चयन कीजिए यदि v = बोहर कक्ष में इलेक्ट्रॉन का वे...
Text Solution
|
- एक अकेला इलेक्ट्रॉन एक स्थायी नामिक की कक्षा में है । नाभिक का आवेश Ze...
Text Solution
|