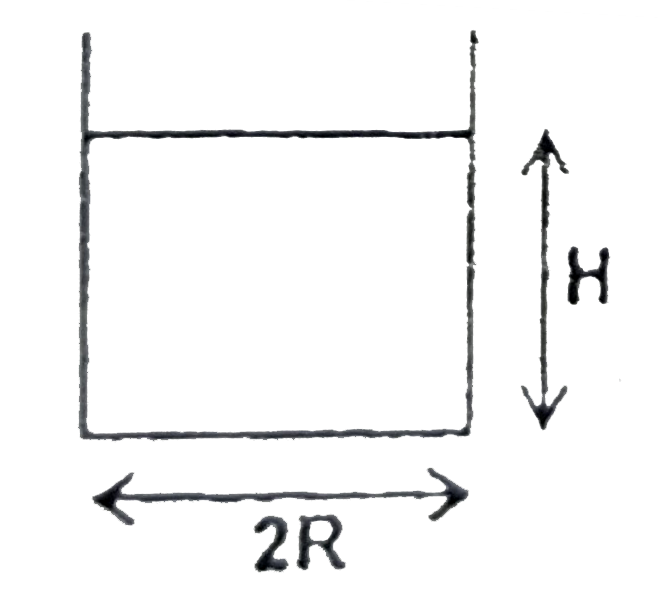
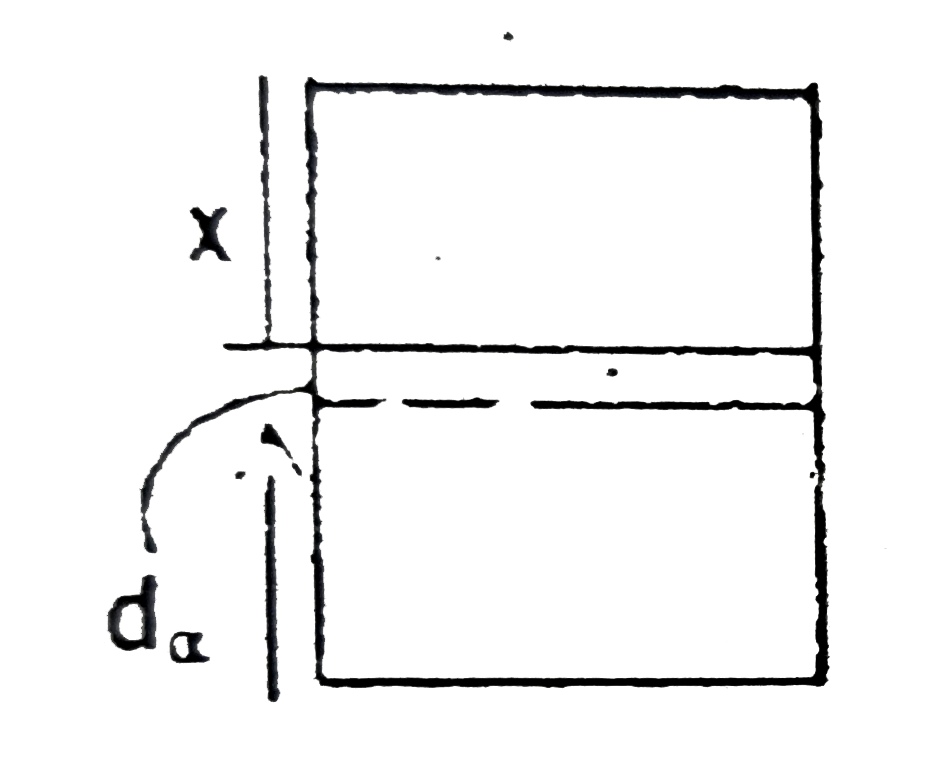
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- On a cold winter dry, the temperature of atmosphere is -T^(@)C. The cy...
Text Solution
|
- On a winter day when the atmospheric temperature drops to -10^(@)C , i...
Text Solution
|
- A layer of ice of thickness y is on the surface of a lake. The air is ...
Text Solution
|
- A small quantity mass m, of water at a temperature theta ("in " ^(@)C)...
Text Solution
|
- On a winter day when the atmospheric temperature drops to -10^(@)C, ic...
Text Solution
|
- Calculate the quantity of heat released during the conversion of10g of...
Text Solution
|
- An ice layer of thickness d1 is floating on a pond of water. The atmos...
Text Solution
|
- The temperature of the water of pond is 0^@ C while that of the surro...
Text Solution
|
- M g of ice at 0^@C is to be converted to water at 0^@C. If L is the la...
Text Solution
|