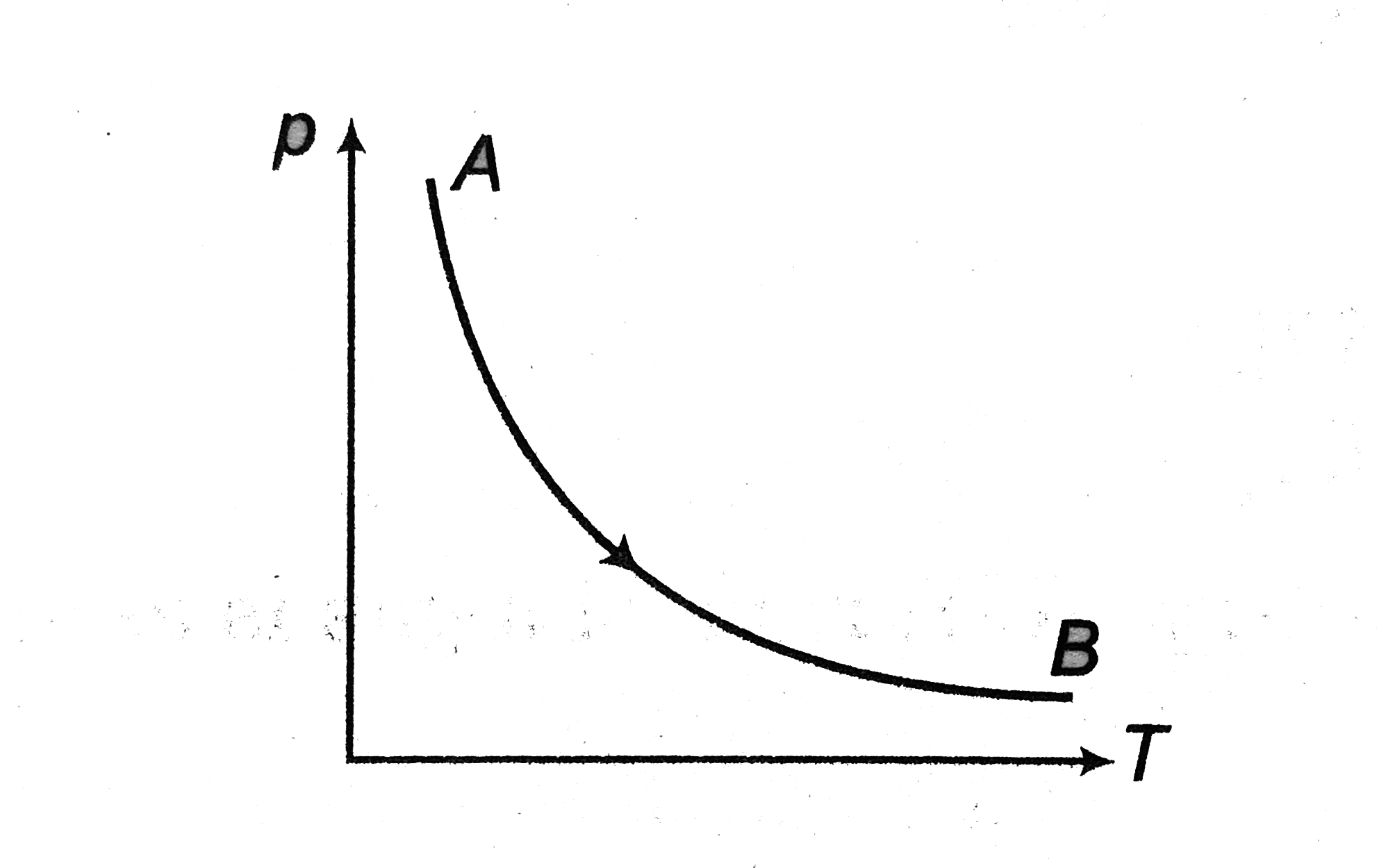
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Two moles of an ideal monoatomic gas are expanded according to the equ...
Text Solution
|
- One mole of an ideal monoatomic gas is taken at a temperature of 300 K...
Text Solution
|
- One mole of an ideal monoatomic gas is initially at 300K. Find the fin...
Text Solution
|
- Two moles of an ideal monoatomic gas are expanded according to the equ...
Text Solution
|
- Three moles of an ideal gas being initially at a temperature Ti=273K w...
Text Solution
|
- If n moles of an ideal gas are expanded is isothermally and reversibly...
Text Solution
|
- An ideal monoatomic gas is adiabatically compressed so that its final ...
Text Solution
|
- An ideal gas is in a container of volume V0 at pressure P0 If the same...
Text Solution
|
- One mole of an ideal monoatomic gas is taken at a temperature of 300 K...
Text Solution
|