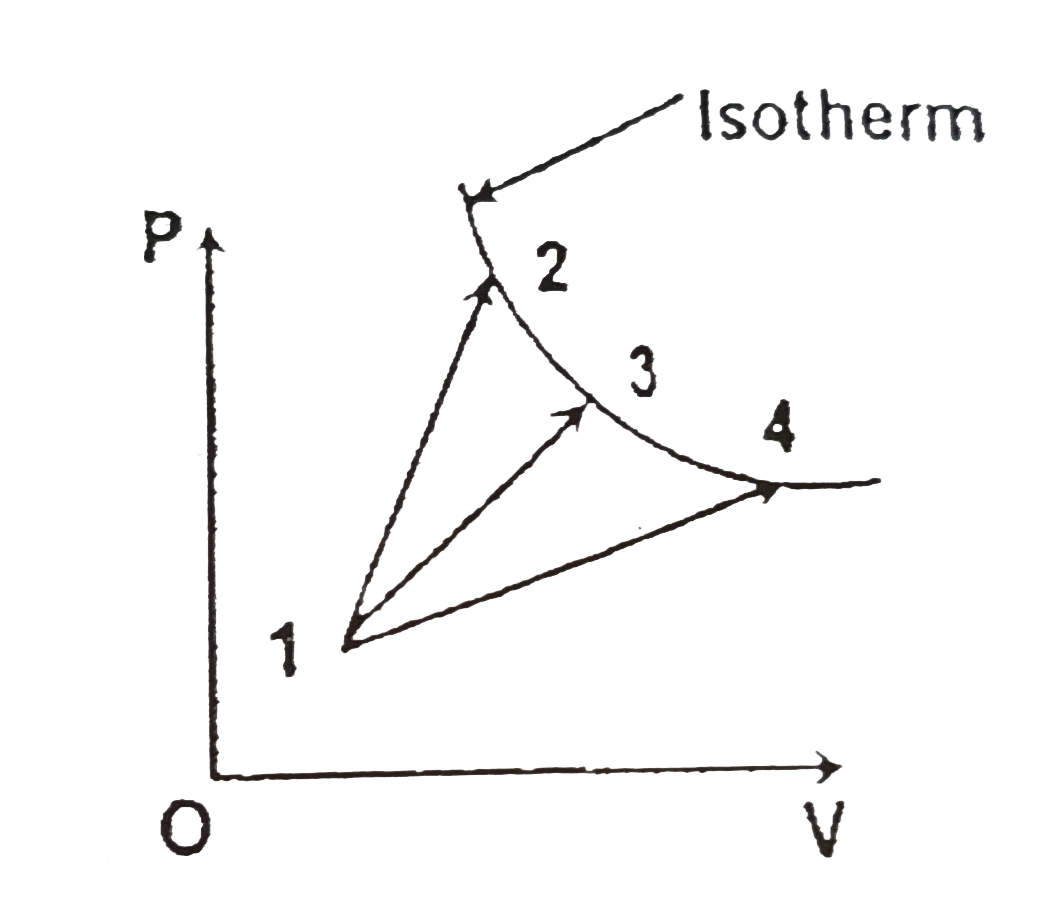
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A gas took part in three thermal processes in which it is heated from ...
Text Solution
|
- A gas takes part in two processes in which it is heated from the same ...
Text Solution
|
- An ideal gas of mass m in a state A goes to another state B via three ...
Text Solution
|
- A gas take part in two thermal processes in which it is heated from th...
Text Solution
|
- A gas take part in two thermal processes in which it is heated from th...
Text Solution
|
- An ideal gas goes from State A to state B via three different process ...
Text Solution
|
- An ideal gas, in initial state 1(P(1), V(1), T(1)) is cooled to a stat...
Text Solution
|
- For an ideal gas four processes are marked as 1,2,3 and 4 on P-V diagr...
Text Solution
|
- One mole of helium gas follows the cycle 1-2-3-1 shown in the diagram....
Text Solution
|