A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
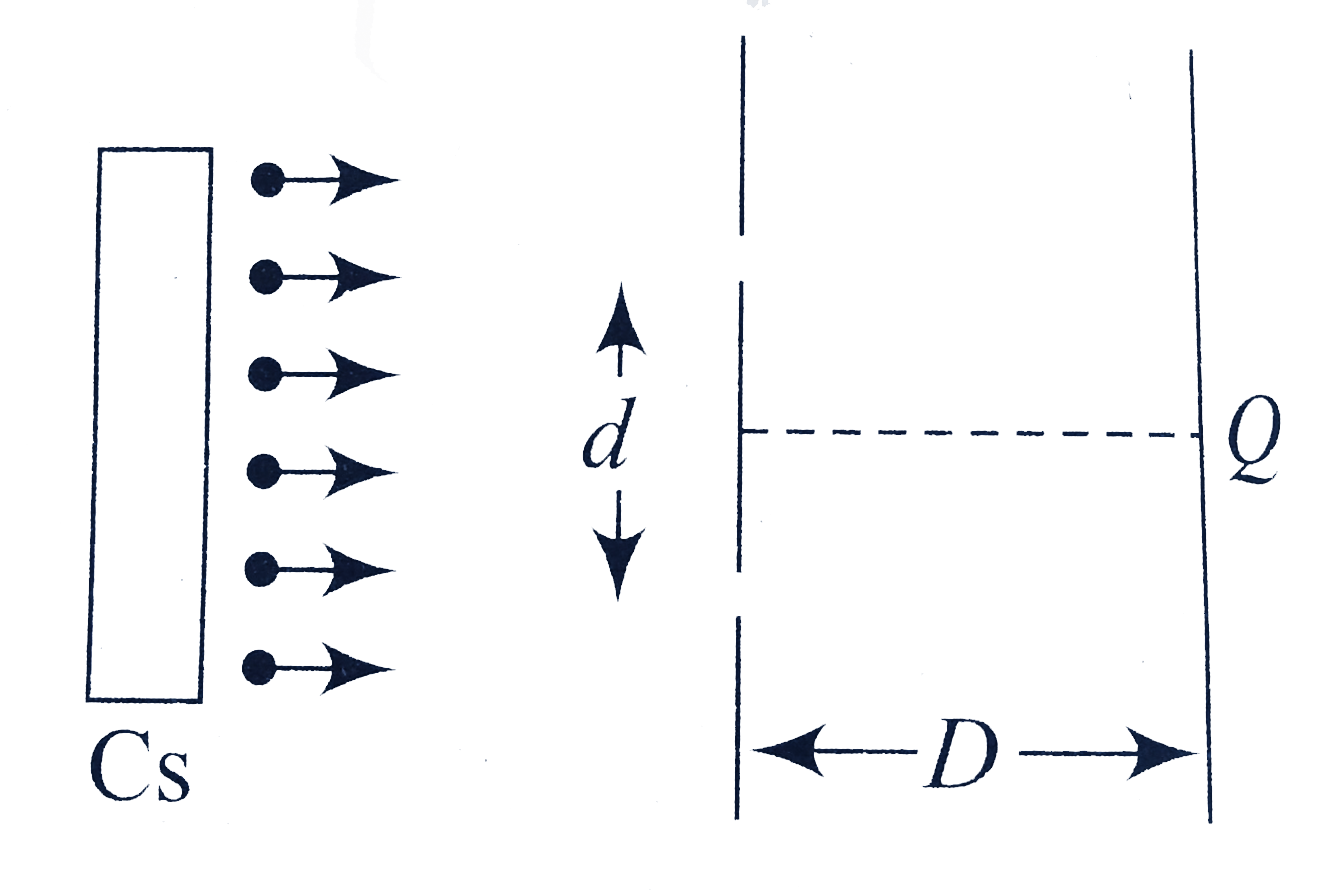
- A Cs plate is irradiated with a light of wavelength lamda=(hc)/(phi),p...
Text Solution
|
- In a photoelectric experiment, the collector plate is at 2.0 V with re...
Text Solution
|
- A Cs plate is irradiated with a light of wavelength lamda=(hc)/(phi) ,...
Text Solution
|
- A Cs plate is irradiated with a light of wavelength lamda=(hc)/(phi) ,...
Text Solution
|
- A Cs plate is irradiated with a light of wavelength lamda=(hc)/(phi) ,...
Text Solution
|
- A very large metal plate carries a charge of Q=-1C . The work function...
Text Solution
|
- If the work function of a metal is 'phi' and the frequency of the inci...
Text Solution
|
- Photon of energy 2.0 eV and wavelength lamdafall on a metal plate and ...
Text Solution
|
- A metal plate of work function phi is illuminated by a monochromatic l...
Text Solution
|