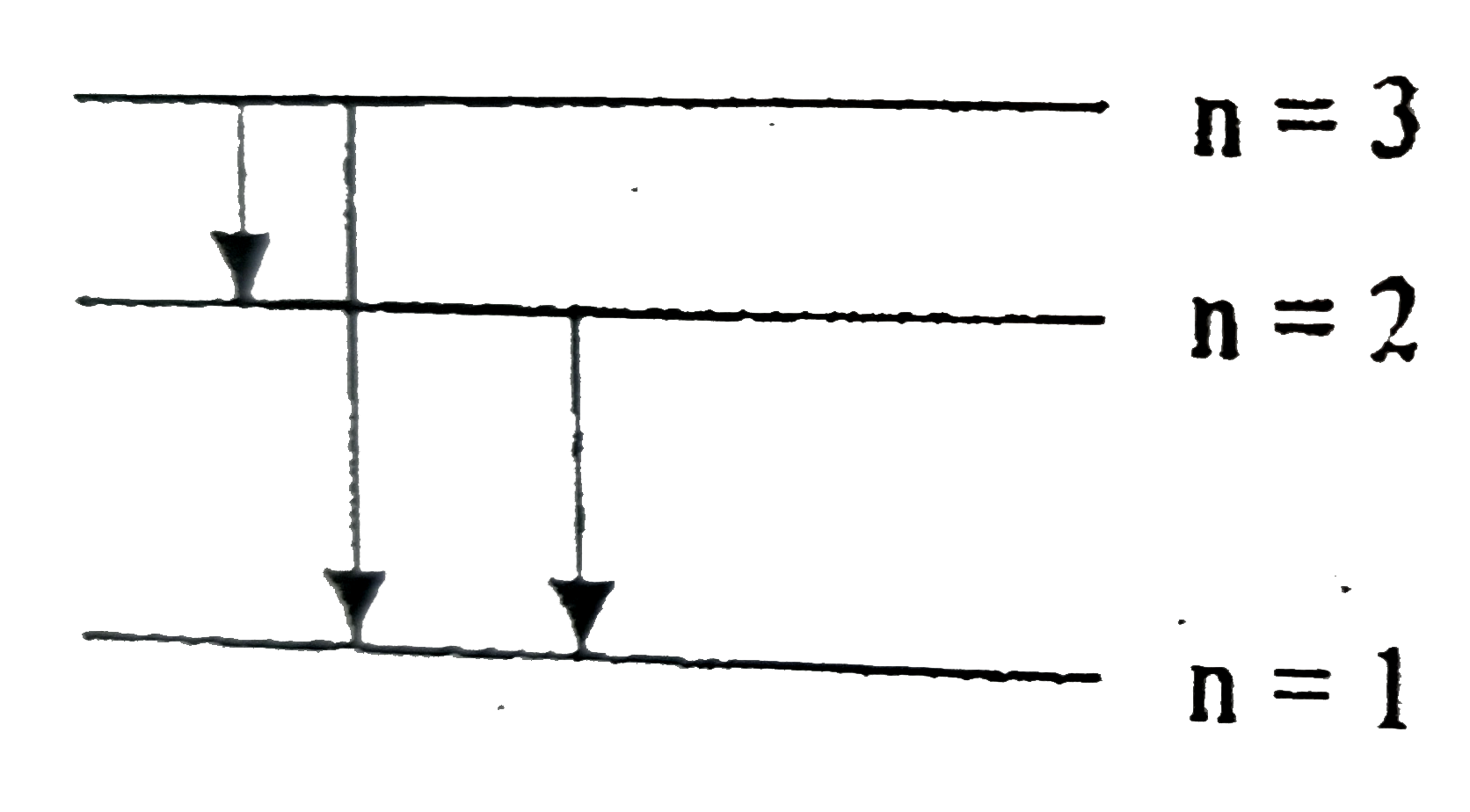
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A gas of hydrogen like atoms can absorb radiation of 68 eV. Consequent...
Text Solution
|
- An atom absorbs a photon of wavelength 500nm and emits another photon ...
Text Solution
|
- Hydrogen atom absorbs radiations of wavelength lambda0 and consequentl...
Text Solution
|
- A gas hydrogen like atoms can absorb radiations of 68eV. Consequently,...
Text Solution
|
- Monochromatic radiation of wavelength lambda is incident on a hydrogen...
Text Solution
|
- Monochromatic radiation of wavelength lambda is incident on a hydrogen...
Text Solution
|
- A sample of H-atoms in ground state absorb the electromagnetic radiati...
Text Solution
|
- A gas of hydrogen like atoms are in an unknown energy state of princip...
Text Solution
|
- A gas of hydrogen like atoms are in an unknown energy state of princip...
Text Solution
|