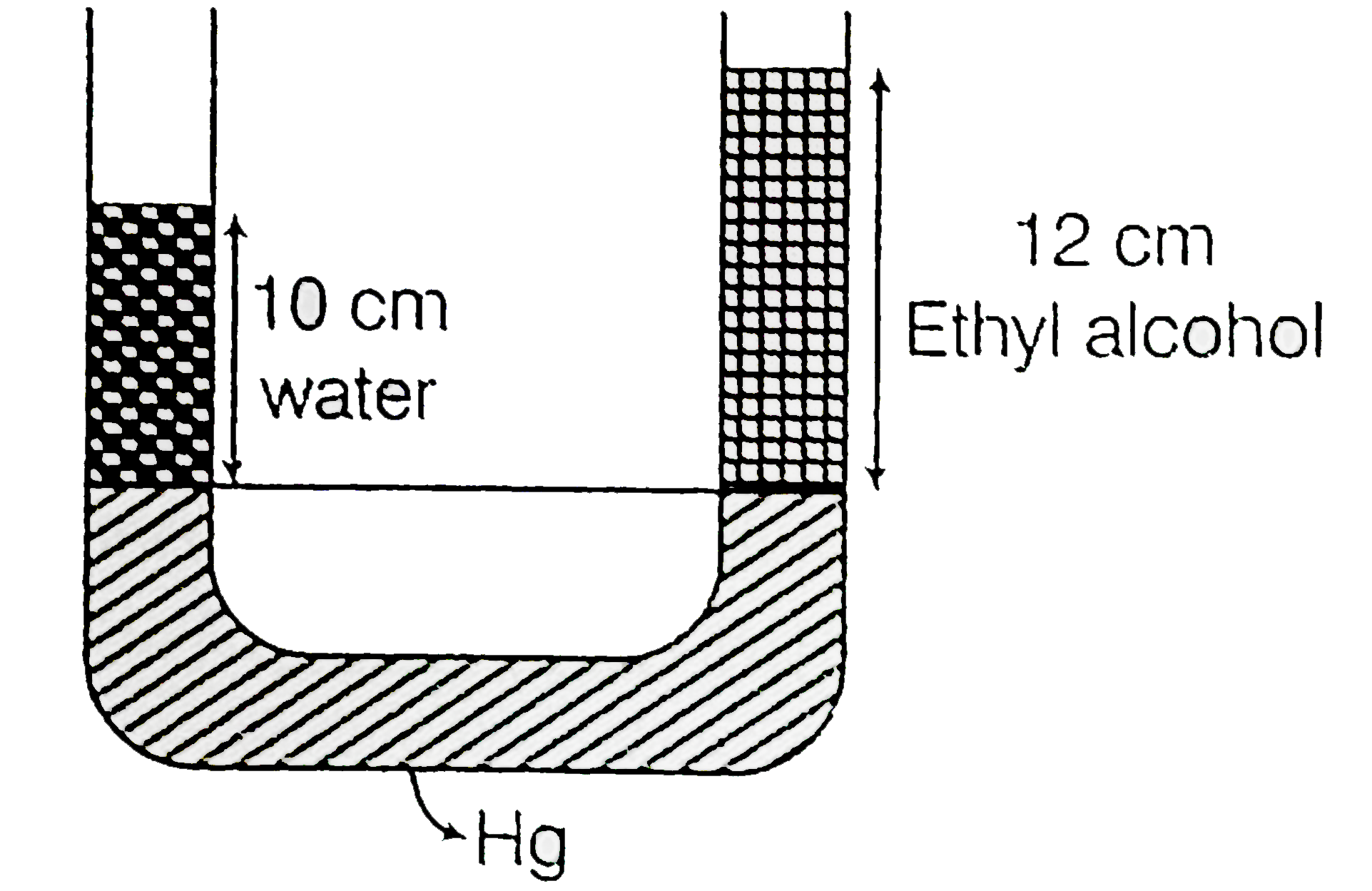Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Find density of ethyl alcohol
Text Solution
|
- Find density of ethyl alcohol
Text Solution
|
- The vapour density of ethyl alcohol vapour is
Text Solution
|
- What is the volume of ethyl alcohol (density 1.15 g/cc) that has to be...
Text Solution
|
- If 40 g of ethyl alcohol is dissolved in 50 mL of water, then calculat...
Text Solution
|
- एथिल ब्रोमाइड से एथिल ऐल्कोहॉल ।
Text Solution
|
- 92 ग्राम एथिल एल्कोहॉल तथा 72 ग्राम जल के विलयन में एथिल एल्कोहॉल...
Text Solution
|
- कैसे प्राप्त करोगे? एथिल ऐमीन से एथिल ऐल्कोहॉल।
Text Solution
|
- कैसे प्राप्त करेंगे --- एथिल एल्कोहॉल से एथिल एमीन
Text Solution
|