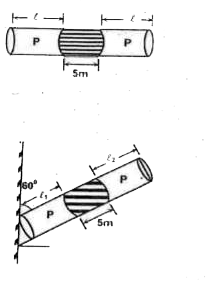
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A thin tube of uniform cross-section is sealed at both ends. It lies h...
Text Solution
|
- A thin tube of uniform cross section is sealed at both ends. It lies h...
Text Solution
|
- A column of mercury of 10cm length is contained in the middle of a nar...
Text Solution
|
- A thin tube of uniform cross section is sealed at both ends. It lies h...
Text Solution
|
- A mercury thread of length 10 cm is contained in the middle of a narro...
Text Solution
|
- एकसमान अनुप्रस्थ - काट की एक पतली नली को दोनों सिरों पर सील किया गया ह...
Text Solution
|
- A horizontal glass tube, sealed at both ends contains a column of merc...
Text Solution
|
- A horizontal tube of 1m is closed at both ends. A mercury column of 20...
Text Solution
|
- An open glass tube is immersed in mercury in such a way that a length ...
Text Solution
|