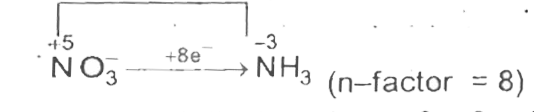
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the reaction given Mg(s)+NO(3)^(ɵ)+H(2)OtoMg(OH)(2)(s)+overset(ɵ)...
Text Solution
|
- BrO(3)^(ɵ) + 5Br^(ɵ) rarr Br(2) + 3 H(2) O IF 50 mL 0.1 M BrO(3)^(ɵ) i...
Text Solution
|
- When 100 " mL of " 0.06 M Fe (NO3)3,50mL of 0.2M FeCl3 and 100mL of 0....
Text Solution
|
- In the reaction given Mg(s)+NO(3)^(ɵ)+H(2)OtoMg(OH)(2)(s)+overset(ɵ)...
Text Solution
|
- 2.0 g sample of NaCN is dissolved in 50 " mL of " 0.3 M mild alkaline ...
Text Solution
|
- 20 " mL of " (M)/(60) KBrO3 was reacted with a sample of SeO(3)^(2-) 2...
Text Solution
|
- 20 " mL of " (M)/(60) KBrO3 was reacted with a sample of SeO(3)^(2-) 2...
Text Solution
|
- 36 " mL of " 0.5 M Br(2) solution when made alkaline undergoes complet...
Text Solution
|
- Which of the following are isoelectronic? NO(3)^(ɵ), CO(3)^(2-), ClO...
Text Solution
|