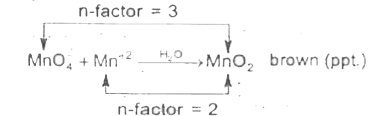
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- H(2) O(2) solution (20 mL) reacts quantitatively with a solution of KM...
Text Solution
|
- 10 " mL of " a solution of H(2)O(2) of 10 violume strength decolourise...
Text Solution
|
- Hydrogen peroxide solution (20 mL) reacts quantitatively with a soluti...
Text Solution
|
- What volume of O(2) measured at standard condition will be formed by t...
Text Solution
|
- A mixture of H(2)C(2)O(4) and NaHC(2)O(4) weighing 2.02 g was dissolve...
Text Solution
|
- 10 mL of a H(2)SO(4) solution is diluted to 100 mL. 25 mL of this dilu...
Text Solution
|
- A solution of H(2)O(2) labelled as '20 V' was left open. Due to this s...
Text Solution
|
- Hydrogen peroxide solution (20 mL) reacts quantitatively with a soluti...
Text Solution
|
- The volume of 10 volume H(2)O(2) solution that decolourises 200 mL of ...
Text Solution
|