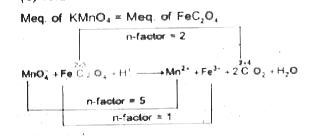
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- What volume of 0.1M KMnO(4) is needed to oxidize 100 mg of FeC(2)O(4)...
Text Solution
|
- What volume of 0.05 M K(2)Cr(2)O(7) in acidic medium is needed for com...
Text Solution
|
- 1 mole each of FeC(2)O(4) and FeSO(4) is oxidised Calculate by 1M KMnO...
Text Solution
|
- How many moles of KMnO(4) are needed to oxidise a mixture of 1 mole of...
Text Solution
|
- What volume of 0.05 M Cr(2)O(7)^(2-) in acid medium is needed for comp...
Text Solution
|
- When 1 mole of KMnO(4) is reacted with FeC(2)O(4) in acidic medium, th...
Text Solution
|
- 0.144 g of pure FeC(2)O(4) was dissolvedin dilute H(2)SO(4) and the so...
Text Solution
|
- One mole of FeC(2)O(4) is oxidised by KMnO(4) in acidic medium. Number...
Text Solution
|
- 100 mL of NaHC(2)O(4) required 50 mL of 0.1M KMnO(4) solution in acidi...
Text Solution
|