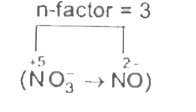
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The volume of 3M HNO(3) required to oxidised 8 g Fe^(2+) is (HNO(...
Text Solution
|
- What volume of 3 molar HNO(3) is needed to oxidise 8 g of Fe^(3+) , HN...
Text Solution
|
- The ratio of coefficient of HNO(3), Fe(N)(3))(2) and NH(4)NO(3) in the...
Text Solution
|
- Find number of species which produce elemental sulphur when treated wi...
Text Solution
|
- [Fe(H(2)O)(6)]Cl(3) में Fe की ऑक्सीकरण संख्या है -
Text Solution
|
- Balance the following redox reaction (i) SnO(2)+c toSn+CO (ii) Fe(3)O(...
Text Solution
|
- Balance the following redox reaction (i) SnO(2)+c toSn+CO (ii) Fe(3)O(...
Text Solution
|
- What volume of3MHNO(3) is needed to oxidize 8 gFe(2+)toFe(3+),HNO(3) ...
Text Solution
|
- What volume of 3 molar HNO(3) is needed to oxidise 8 g of Fe^(3+), HNO...
Text Solution
|