A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
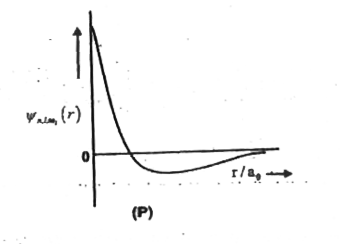
- The wave function psi(n,l,m) is a mathematical function whose value de...
Text Solution
|
- The wave function, psi(n), l, m(l) is a mathematical function whose va...
Text Solution
|
- The wave function, psi(n), l, m(l) is a mathematical function whose va...
Text Solution
|
- One of the major requirement in atomic structure is determination of l...
Text Solution
|
- One of the major requirement in atomic structure is determination of l...
Text Solution
|
- The wave function Phi(n,l,m(1)) is a methematical function whose value...
Text Solution
|
- The wave function Phi(n,l,m(1)) is a methematical function whose value...
Text Solution
|
- The wave function Phi(n,l,m(1)) is a methematical function whose value...
Text Solution
|
- The Schrodinger wave equation for H-atom is nabla^(2) Psi = (8pi^(2)m)...
Text Solution
|