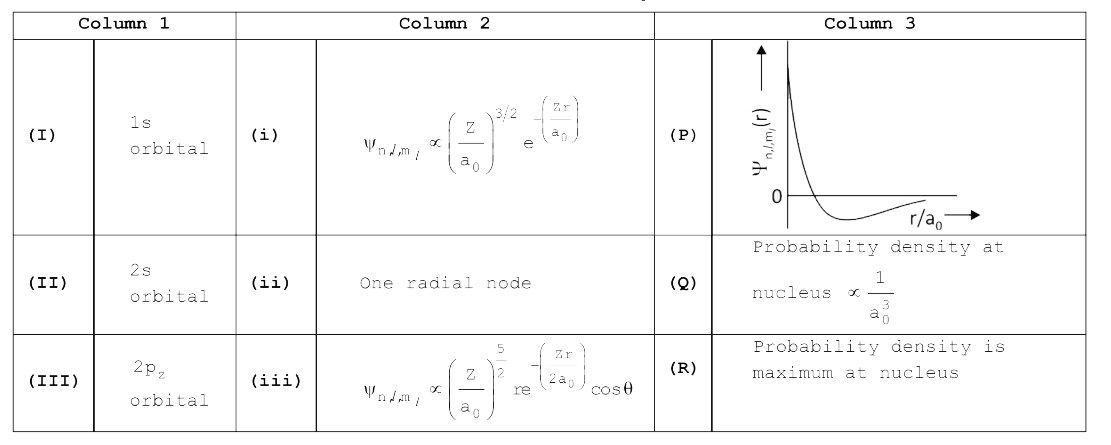
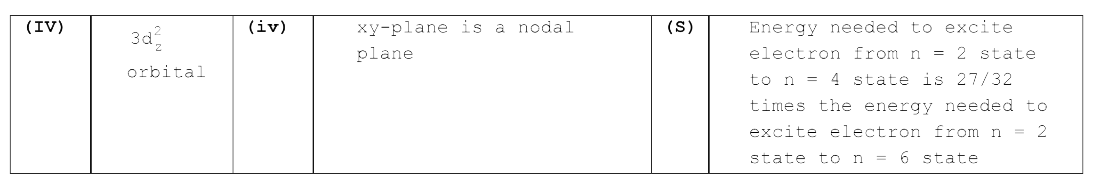A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The wave function, Psi("n, l, m(l)) is a mathematical function whose...
Text Solution
|
- Radial wave function depends on the quantum numbers (i) n (ii) l (...
Text Solution
|
- The wave function, psi(n), l, m(l) is a mathematical function whose va...
Text Solution
|
- The wave function, psi(n), l, m(l) is a mathematical function whose va...
Text Solution
|
- The wave function, psi(n), l, m(l) is a mathematical function whose va...
Text Solution
|
- The wave function Phi(n,l,m(1)) is a methematical function whose value...
Text Solution
|
- The wave function Phi(n,l,m(1)) is a methematical function whose value...
Text Solution
|
- The wave function Phi(n,l,m(1)) is a methematical function whose value...
Text Solution
|
- Wave function psi(n,lm(1)) is a mathematical function, which vlaue dep...
Text Solution
|