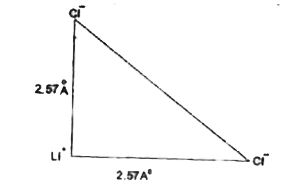
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The edge length of LiCl (NaCl structure) unit cell 514 pm. Assuming an...
Text Solution
|
- The unit cube length for LiCl (NaCl structure) is 5.14 Å. Assuming ani...
Text Solution
|
- The unit cell cube length for LiCl(just like NaCl structures) is 5.14Å...
Text Solution
|
- The unit cube length for LiCl(NaCl structure) is 5.14overset(@)A . Ass...
Text Solution
|
- The unit cell edge length of NaF crystal is 4.634Å. If the ionic radiu...
Text Solution
|
- The edge length of LiCl (NaCl structure) unit cell is 514 pm. Assumi...
Text Solution
|
- The unit cube length for LiCl (NaCl structure) is 5.14 Å. Assuming ani...
Text Solution
|
- Assertion. If the length of the unit cell of LiCl having NaCl structur...
Text Solution
|
- The unit cube length for LiCl (NaCl structure) is 5.14 Å. Assuming ani...
Text Solution
|