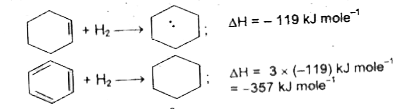Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the enthalpy of combustion of benzene (l) on the basis of th...
Text Solution
|
- Calculate the resonance energy of gaseous benzene form the following d...
Text Solution
|
- Calculate the resonance enegry of toulene (use Kekule structure form t...
Text Solution
|
- The enthalpy change for the combustion of N(2)H(4)(l) (Hydrazine) is -...
Text Solution
|
- Calculate the enthalpy of combustion of benzene (l) on the basis of th...
Text Solution
|
- Find DeltaH of the process NaOH(s) rarr NaOH(g) Given: Delta(diss)H^(T...
Text Solution
|
- Delta(f)H^(Theta) of Cyclohexene (l) and benzene at 25^(@)C is -156 an...
Text Solution
|
- The combustion of 1 mol of benzene takes place at 298 K and 1 atm . Af...
Text Solution
|
- Delta(f)H^(Theta) of Cyclohexene (l) and benzene at 25^(@)C is -156 an...
Text Solution
|