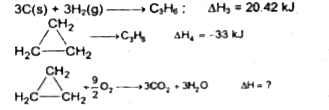Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- Standard enthalpy of combustion of cyclopropane is -2091 kJ//"mole" at...
Text Solution
|
- Calculate the enthalpy of formation of acetic acid (CH(3)COOH) if its ...
Text Solution
|
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- The enthalpy of combustion of methane, graphite and dihydrogen at 298 ...
Text Solution
|
- Calculate the value of enthalpy of combustion of cyclopropane at 25^(@...
Text Solution
|
- The enthalpy of combustion of methane, graphite and dihydrogen at 298K...
Text Solution
|
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|