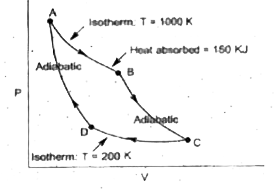
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The accompanying represents a reversible camot cycle for an ideal gas:...
Text Solution
|
- The change in entropy of an ideal gas during reversible isothermal exp...
Text Solution
|
- During an adiabatic reversibly expansion of an ideal gas
Text Solution
|
- For isothemal expansion in case of an ideal gas:
Text Solution
|
- The accompanying diagram represents a reversible cannot cycle for an i...
Text Solution
|
- The accompanying diagram represents a reversible cannot cycle for an i...
Text Solution
|
- The accompanying diagram represents a reversible cannot cycle for an i...
Text Solution
|
- During an isothermal reversible expansion of an ideal gas, its
Text Solution
|
- The accompanying diagram represents a reversible cannot cycle for an i...
Text Solution
|