
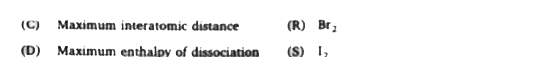
Text Solution
Verified by Experts
`(A) to (Q), DeltaS_("total") gt 0` is according to second law of thermodynamics it states entropy of universe is continuously increasing due to spontaneous processes taking place in it.
`(B) to ( R)`, If `DeltaS_("total") lt 0`, the process is non-spontaneous.
`DeltaS_("total")` means `DeltaS_("sys") + DeltaS_("surr") gt 0`, for a spontaneous process.
`( C) to (S), DeltaH = DeltaU + DeltanRT`
`(D) to (P), =-DeltaG = W_("useful")`, i.e. useful work done by the system.
i.e. decreases in free energy is measure of useful work done by the system.
`(B) to ( R)`, If `DeltaS_("total") lt 0`, the process is non-spontaneous.
`DeltaS_("total")` means `DeltaS_("sys") + DeltaS_("surr") gt 0`, for a spontaneous process.
`( C) to (S), DeltaH = DeltaU + DeltanRT`
`(D) to (P), =-DeltaG = W_("useful")`, i.e. useful work done by the system.
i.e. decreases in free energy is measure of useful work done by the system.