A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
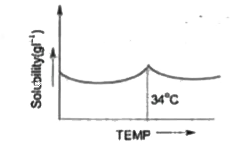
- Solubility curve of Na(2)SO(4).10H(2)O in water with temperature is gi...
Text Solution
|
- Amount of oxygen in 32.2 g of Na(2)SO(4).10H(2)O is:
Text Solution
|
- 1.61 gm of Na(2)SO(4). 10H(2)O contains same number of oxygen atoms as...
Text Solution
|
- 40.25g of Glauber's salt (Na(2)SO(4).10H(2)O) is dissolved in water to...
Text Solution
|
- Na(2)b(4)O(7)10H(2)O is
Text Solution
|
- Na(2)SO(4) is water soluble but BaSO(4) is insoluble because :
Text Solution
|
- At what relative humidity with Na(2)SO(4) be deliquescent (absorb mois...
Text Solution
|
- Statement-1: Heat of solution is positive when Na(2)SO(4)*10H(2)O is d...
Text Solution
|
- Na(2)SO(4).10H(2)O(s)hArrNa(2)SO(4).5H(2)O(g) K(P)=2.43xx10^(-10) atm^...
Text Solution
|